| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2018-05-02 12:58:43 UTC |
|---|
| Update date | 2018-05-04 14:08:35 UTC |
|---|
| FoodComEx ID | PC001229 |
|---|
| FoodDB Record | FDB022331 |
|---|
| Chemical Information |
|---|
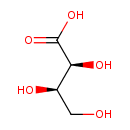
| Name | Threonic acid |
|---|
| Description | Threonic acid also known as threonate, belongs to the class of organic compounds known as sugar acids. Sugar acids are compounds containing a saccharide unit which bears a carboxylic acid group. Threonic acid is a weak acid (based on its pKa). Threonic acid is derived from threose. The L-isomer is a metabolite of ascorbic acid (vitamin C). Threonic acid is probably derived from glycated proteins or from the degradation of ascorbic acid. It is a normal component is aqueous humour and blood (PMID: 10420182). One recent study suggested that because L-threonate inhibits DKK1 expression in vitro, it may have potential in the treatment of androgenic alopecia (PMID: 21034532). Threonic acid is a substrate of L-threonate 3-dehydrogenase (EC 1.1.1.129) in the ascorbate and aldarate metabolism pathway (KEGG). Threonate has also been found to be a microbial metabolite (PMID: 20615997). |
|---|
| CAS Number | 3909-12-4 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (R*,S*)-2,3,4-trihydroxy-Butanoate | hmdb | | (R*,S*)-2,3,4-trihydroxy-Butanoic acid | hmdb | | threo-2,3,4-Trihydroxybutyrate | hmdb | | threo-2,3,4-Trihydroxybutyric acid | hmdb | | threonate | hmdb |
|
|---|
| Chemical Formula | C4H8O5 |
|---|
| IUPAC name | (2S,3R)-2,3,4-trihydroxybutanoic acid |
|---|
| InChI Identifier | InChI=1S/C4H8O5/c5-1-2(6)3(7)4(8)9/h2-3,5-7H,1H2,(H,8,9)/t2-,3+/m1/s1 |
|---|
| InChI Key | JPIJQSOTBSSVTP-GBXIJSLDSA-N |
|---|
| Isomeric SMILES | OC[C@@H](O)[C@H](O)C(O)=O |
|---|
| Average Molecular Weight | 136.1033 |
|---|
| Monoisotopic Molecular Weight | 136.037173366 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-hydroxy acid
- Short-chain hydroxy acid
- Sugar acid
- Alpha-hydroxy acid
- Hydroxy acid
- Monosaccharide
- Fatty acid
- Secondary alcohol
- Polyol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Alcohol
- Carbonyl group
- Primary alcohol
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | 1 to 2 mg |
|---|
| Delivery Time | 2 weeks |
|---|
| Storage Form | powder |
|---|
| Storage Conditions | -18°C |
|---|
| Stability | Not Available |
|---|
| Purity | unknown |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
| Contact Name | Contact Institution | Contact Email |
|---|
| Augustin Scalbert | International Agency for Research on Cancer (IARC), Biomarkers Group, 150 cours Albert Thomas, Lyon, FR, 69372 | scalberta@iarc.fr |
|
| Commercial Vendors |
|---|
| Not Available |