| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2018-05-02 12:51:02 UTC |
|---|
| Update date | 2018-05-04 14:14:14 UTC |
|---|
| FoodComEx ID | PC001216 |
|---|
| FoodDB Record | FDB012295 |
|---|
| Chemical Information |
|---|
| Name | Piceatannol |
|---|
| Description | Piceatannol, also known as (Z)-3,5,3',4'-tetrahydroxystilbene, is a member of the class of compounds known as stilbenes. Stilbenes are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. Piceatannol is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). Piceatannol can be synthesized from cis-stilbene. Piceatannol can also be synthesized into cis-astringin. Piceatannol can be found in common grape and grape wine, which makes piceatannol a potential biomarker for the consumption of these food products. Piceatannol is a stilbenoid, a type of phenolic compound . |
|---|
| CAS Number | 4339-71-3 |
|---|
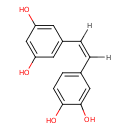
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 3,3',4,5'-Stilbenetetrol, 8CI | db_source | | 3,3',4,5'-Tetrahydroxystilbene | db_source | | 4-[2-(3,5-Dihydroxyphenyl)ethenyl]-1,2-benzenediol, 9CI | db_source | | Astringenin | db_source | | NSC 365798 | db_source | | Piceatannol | db_source |
|
|---|
| Chemical Formula | C14H12O4 |
|---|
| IUPAC name | 4-[(Z)-2-(3,5-dihydroxyphenyl)ethenyl]benzene-1,2-diol |
|---|
| InChI Identifier | InChI=1S/C14H12O4/c15-11-5-10(6-12(16)8-11)2-1-9-3-4-13(17)14(18)7-9/h1-8,15-18H/b2-1- |
|---|
| InChI Key | CDRPUGZCRXZLFL-UPHRSURJSA-N |
|---|
| Isomeric SMILES | [H]\C(=C(/[H])C1=CC(O)=CC(O)=C1)C1=CC=C(O)C(O)=C1 |
|---|
| Average Molecular Weight | 244.246 |
|---|
| Monoisotopic Molecular Weight | 244.073558866 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- Styrene
- Resorcinol
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | 1 to 2 mg |
|---|
| Delivery Time | 2 weeks |
|---|
| Storage Form | powder |
|---|
| Storage Conditions | 4°C |
|---|
| Stability | Not Available |
|---|
| Purity | unknown |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
| Contact Name | Contact Institution | Contact Email |
|---|
| Augustin Scalbert | International Agency for Research on Cancer (IARC), Biomarkers Group, 150 cours Albert Thomas, Lyon, FR, 69372 | scalberta@iarc.fr |
|
| Commercial Vendors |
|---|
| Not Available |