| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2018-05-02 12:32:42 UTC |
|---|
| Update date | 2018-05-04 14:22:47 UTC |
|---|
| FoodComEx ID | PC001198 |
|---|
| FoodDB Record | FDB022597 |
|---|
| Chemical Information |
|---|
| Name | Hydroxycotinine |
|---|
| Description | Quantitatively, the most important metabolite of nicotine in most mammalian species is cotinine. In humans, about 70 to 80% of nicotine is converted to cotinine. 3-Hydroxycotinine (3HC) is the main nicotine metabolite detected in smokers urine. It is also excreted as a glucuronide conjugate (3HC-Gluc). 3HC and 3HC-Gluc account for 40-60% of the nicotine dose in urine. [HMDB] |
|---|
| CAS Number | 34834-67-8 |
|---|
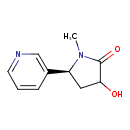
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (3R-trans)-3-hydroxy-1-methyl-5-(3-pyridinyl)-2-Pyrrolidinone | hmdb | | 3-Hydroxy-1-methyl-5-(3-pyridinyl)-2-pyrrolidinone | hmdb | | 3-Hydroxycotinine | hmdb | | trans-3-Hydroxycotinine | hmdb | | trans-3'-hydroxycotinine | hmdb |
|
|---|
| Chemical Formula | C10H12N2O2 |
|---|
| IUPAC name | (5S)-3-hydroxy-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one |
|---|
| InChI Identifier | InChI=1S/C10H12N2O2/c1-12-8(5-9(13)10(12)14)7-3-2-4-11-6-7/h2-4,6,8-9,13H,5H2,1H3/t8-,9?/m0/s1 |
|---|
| InChI Key | XOKCJXZZNAUIQN-IENPIDJESA-N |
|---|
| Isomeric SMILES | CN1[C@@H](CC(O)C1=O)C1=CN=CC=C1 |
|---|
| Average Molecular Weight | 192.2145 |
|---|
| Monoisotopic Molecular Weight | 192.089877638 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrrolidinylpyridines. Pyrrolidinylpyridines are compounds containing a pyrrolidinylpyridine ring system, which consists of a pyrrolidine ring linked to a pyridine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyrrolidinylpyridines |
|---|
| Direct Parent | Pyrrolidinylpyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrolidinylpyridine
- Alkaloid or derivatives
- Pyrrolidone
- 2-pyrrolidone
- N-alkylpyrrolidine
- Pyrrolidine
- Tertiary carboxylic acid amide
- Heteroaromatic compound
- Carboxamide group
- Lactam
- Secondary alcohol
- Carboxylic acid derivative
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | 1 to 2 mg |
|---|
| Delivery Time | 2 weeks |
|---|
| Storage Form | powder |
|---|
| Storage Conditions | -18°C |
|---|
| Stability | Not Available |
|---|
| Purity | unknown |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
| Contact Name | Contact Institution | Contact Email |
|---|
| Augustin Scalbert | International Agency for Research on Cancer (IARC), Biomarkers Group, 150 cours Albert Thomas, Lyon, FR, 69372 | scalberta@iarc.fr |
|
| Commercial Vendors |
|---|
| AKSci | X3867 |
|---|
| Cayman Chemical | 16100 |
|---|
| Toronto Research Chemicals | H924500 |
|---|
| Toronto Research Chemicals | KIT0550 |
|---|