| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2018-05-02 12:32:02 UTC |
|---|
| Update date | 2018-05-04 14:22:51 UTC |
|---|
| FoodComEx ID | PC001197 |
|---|
| FoodDB Record | FDB001819 |
|---|
| Chemical Information |
|---|
| Name | Hippuric acid |
|---|
| Description | Hippuric acid (Gr. hippos, horse, ouron, urine) is a carboxylic acid found in the urine of horses and other herbivores. Hippuric acid crystallizes in rhombic prisms which are readily soluble in hot water, melt at 187 °C and decompose at about 240 °C. High concentrations of hippuric acid can also indicate a toluene intoxication. When many aromatic compounds such as benzoic acid and toluene are taken internally, they are converted to hippuric acid by reaction with the amino acid, glycine.; Hippuric acid is an acyl glycine formed by the conjugation of benzoic aicd with glycine. Acyl glycines are produced through the action of glycine N-acyltransferase (EC 2.3.1.13) which is an enzyme that catalyzes the chemical reaction: acyl-CoA + glycine < -- > CoA + N-acylglycine. Hippuric acid is a normal component of urine and is typically increased with increased consumption of phenolic compounds (tea, wine, fruit juices). These phenols are converted to benzoic acid which is then converted to hippuric acid and excreted in the urine. Hippuric acid is the most frequently used biomarker in the biological monitoring of occupational exposure to toluene. This product of solvent biotransformation may be also found in the urine of individuals who have not been exposed to the solvent. A smaller fraction of the absorbed toluene is oxidized to aromatic compounds including ortho-cresol, which is not found significantly in the urine of nonexposed individuals. The concentration of hippuric acid in the urine of individuals exposed to a low toluene concentration does not differ from that of individuals not exposed to the solvent. This has led to the conclusion that hippuric acid should not be utilized in the biological monitoring of occupational exposure to low levels of toluene in the air.; Protein-bound organic acids such as hippuric acid are markedly accumulated in uremic plasma and produce defective protein binding of drugs. (PMID: 9120876, 8734460). |
|---|
| CAS Number | 495-69-2 |
|---|
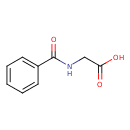
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (benzoylamino)-acetate | biospider | | (benzoylamino)-acetic acid | biospider | | (benzoylamino)acetic acid | biospider | | 2-benzamido-acetic acid | biospider | | 2-Benzamidoacetate | biospider | | 2-Benzamidoacetic acid | biospider | | 532-93-4 (mono-ammonium salt) | biospider | | 532-94-5 (mono-hydrochloride salt) | biospider | | 583-10-8 (mono-potassium salt) | biospider | | Acetic acid, (benzoylamino)- | biospider | | Acetic acid,benzamide hippuric acid | biospider | | Acido ippurico | biospider | | Benzamidoacetate | biospider | | Benzamidoacetic acid | biospider | | Benzaminoacetic acid | biospider | | Benzenecarboxamide, n-carboxymethyl- | biospider | | Benzoylaminoacetic acid | biospider | | Benzoylglycin | biospider | | Benzoylglycine | biospider | | Benzoylglycocoll | biospider | | Glycine, n-benzoyl- | biospider | | Hippurate | biospider | | Hippuricum acidum | biospider | | N-(phenylcarbonyl)glycine | biospider | | N-benzoyl-glycin | biospider | | N-benzoylglycine | biospider | | Phenylcarbonylaminoacetate | biospider | | Phenylcarbonylaminoacetic acid | biospider |
|
|---|
| Chemical Formula | C9H9NO3 |
|---|
| IUPAC name | 2-(phenylformamido)acetic acid |
|---|
| InChI Identifier | InChI=1S/C9H9NO3/c11-8(12)6-10-9(13)7-4-2-1-3-5-7/h1-5H,6H2,(H,10,13)(H,11,12) |
|---|
| InChI Key | QIAFMBKCNZACKA-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(=O)CNC(=O)C1=CC=CC=C1 |
|---|
| Average Molecular Weight | 179.1727 |
|---|
| Monoisotopic Molecular Weight | 179.058243159 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hippuric acids. Hippuric acids are compounds containing hippuric acid, which consists of a of a benzoyl group linked to the N-terminal of a glycine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Hippuric acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hippuric acid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Benzoyl
- Carboxamide group
- Secondary carboxylic acid amide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 0.31 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 3.75 mg/mL at 25 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | 187-191 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | 1 to 2 mg |
|---|
| Delivery Time | 2 weeks |
|---|
| Storage Form | powder |
|---|
| Storage Conditions | RT |
|---|
| Stability | Not Available |
|---|
| Purity | unknown |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
| Contact Name | Contact Institution | Contact Email |
|---|
| Augustin Scalbert | International Agency for Research on Cancer (IARC), Biomarkers Group, 150 cours Albert Thomas, Lyon, FR, 69372 | scalberta@iarc.fr |
|
| Commercial Vendors |
|---|
| AKSci | F131 |
|---|
| AKSci | J93268 |
|---|
| Glentham | GM5145 |
|---|
| MetaSci | HMDB0000714 |
|---|
| Sigma-Aldrich | HMDB0000714 |
|---|
| Toronto Research Chemicals | H356700 |
|---|