| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2018-05-02 12:30:47 UTC |
|---|
| Update date | 2018-05-04 14:23:00 UTC |
|---|
| FoodComEx ID | PC001195 |
|---|
| FoodDB Record | FDB022194 |
|---|
| Chemical Information |
|---|
| Name | Glycoursodeoxycholic acid |
|---|
| Description | Glycoursodeoxycholic acid is an acyl glycine and a bile acid-glycine conjugate. It is a secondary bile acid produced by the action of enzymes existing in the microbial flora of the colonic environment. In hepatocytes, both primary and secondary bile acids undergo amino acid conjugation at the C-24 carboxylic acid on the side chain, and almost all bile acids in the bile duct therefore exist in a glycine conjugated form (PMID:16949895). Bile acids are steroid acids found predominantly in bile of mammals. The distinction between different bile acids is minute, depends only on presence or absence of hydroxyl groups on positions 3, 7, and 12.
Bile acids are physiological detergents that facilitate excretion, absorption, and transport of fats and sterols in the intestine and liver. Bile acids are also steroidal amphipathic molecules derived from the catabolism of cholesterol. They modulate bile flow and lipid secretion, are essential for the absorption of dietary fats and vitamins, and have been implicated in the regulation of all the key enzymes involved in cholesterol homeostasis.
Bile acids recirculate through the liver, bile ducts, small intestine and portal vein to form an enterohepatic circuit. They exist as anions at physiological pH and, consequently, require a carrier for transport across the membranes of the enterohepatic tissues. The unique detergent properties of bile acids are essential for the digestion and intestinal absorption of hydrophobic nutrients. Bile acids have potent toxic properties (e.g., membrane disruption) and there are a plethora of mechanisms to limit their accumulation in blood and tissues. (PMID: 11316487, 16037564, 12576301, 11907135) [HMDB] |
|---|
| CAS Number | 64480-66-6 |
|---|
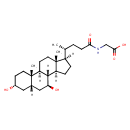
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 3a,7b-Dihydroxy-5b-cholanoylglycine | hmdb | | Glycoursodeoxycholate | hmdb | | Glycoursodeoxycholic acid | hmdb | | Glycylursodeoxycholate | hmdb | | Glycylursodeoxycholic acid | hmdb | | N-(3a,7b-Dihydroxy-5b-cholan-24-oyl)glycine | hmdb | | N-[(3a,5b,7b)-3,7-Dihydroxy-24-oxocholan-24-yl]glycine | hmdb | | Ursodeoxycholylglycine | hmdb |
|
|---|
| Chemical Formula | C26H43NO5 |
|---|
| IUPAC name | 2-[(4R)-4-[(1S,2S,5R,7S,9S,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]acetic acid |
|---|
| InChI Identifier | InChI=1S/C26H43NO5/c1-15(4-7-22(30)27-14-23(31)32)18-5-6-19-24-20(9-11-26(18,19)3)25(2)10-8-17(28)12-16(25)13-21(24)29/h15-21,24,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16+,17-,18-,19+,20+,21+,24+,25+,26-/m1/s1 |
|---|
| InChI Key | GHCZAUBVMUEKKP-XROMFQGDSA-N |
|---|
| Isomeric SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCC(O)=O |
|---|
| Average Molecular Weight | 449.6233 |
|---|
| Monoisotopic Molecular Weight | 449.314123491 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as glycinated bile acids and derivatives. Glycinated bile acids and derivatives are compounds with a structure characterized by the presence of a glycine linked to a bile acid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Glycinated bile acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glycinated bile acid
- Dihydroxy bile acid, alcohol, or derivatives
- Hydroxy bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- Hydroxysteroid
- 7-hydroxysteroid
- 7-alpha-hydroxysteroid
- 3-alpha-hydroxysteroid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Fatty amide
- Fatty acyl
- N-acyl-amine
- Cyclic alcohol
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Organooxygen compound
- Organopnictogen compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Organonitrogen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | 1 to 2 mg |
|---|
| Delivery Time | 2 weeks |
|---|
| Storage Form | powder |
|---|
| Storage Conditions | RT |
|---|
| Stability | Not Available |
|---|
| Purity | unknown |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
| Contact Name | Contact Institution | Contact Email |
|---|
| Augustin Scalbert | International Agency for Research on Cancer (IARC), Biomarkers Group, 150 cours Albert Thomas, Lyon, FR, 69372 | scalberta@iarc.fr |
|
| Commercial Vendors |
|---|
| AKSci | 9870AB |
|---|
| Cayman Chemical | 21698 |
|---|
| Cayman Chemical | HMDB0000708 |
|---|
| Glentham | GL1421 |
|---|
| MetaSci | HMDB0000708 |
|---|
| Toronto Research Chemicals | G679550 |
|---|