| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2018-05-02 12:14:01 UTC |
|---|
| Update date | 2018-05-04 14:23:46 UTC |
|---|
| FoodComEx ID | PC001184 |
|---|
| FoodDB Record | FDB002488 |
|---|
| Chemical Information |
|---|
| Name | Enterolactone |
|---|
| Description | Production of intestinal flora acting on lignans present in cereal
Enterolactone is a mammalian lignan that have a similar biphenolic structure to lignans from plants. Lignans are compounds with estrogenic properties and are probably the most important source of phytoestrogens in western diets. Mammalian lignans are formed from precursors that are contained mainly in vegetables, whole grain products and berries, via action of intestinal microflora. Enterolactone is produced in the colon by the action of bacteria on secoisolariciresinol, matairesinol and its glycosides. Secoisolariciresinol is converted to enterodiol which is subsequently converted to enterolactone as it passes through the colon. Matairesinol is converted directly to enterolactone. Enterolactone have been shown to possess weakly estrogenic and antiestrogenic activities, and it has been suggested that the high production of this antiestrogenic mammalian lignans in the gut may serve to protect against breast cancer in women and prostate cancer in men; however epidemiological evidence to date is conflicting. (PMID: 16168401, 12270221, 11216511, 12107024). Enterolactone is a biomarker for the consumption of soy beans and other soy products. Enterolactone is found in cereals and cereal products. |
|---|
| CAS Number | 76543-15-2 |
|---|
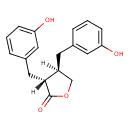
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2,3-Bhbb | biospider | | 2,3-Bis(3-hydroxybenzyl)butyrolactone | db_source | | 2,3-bis(3'-hydroxybenzyl)butyrolactone | biospider | | 2(3H)-Furanone, dihydro-3,4-bis((3-hydroxyphenyl)methyl)- | biospider | | 3,4-Bis((3-hydroxyphenyl)methyl)dihydro-2-(3H)-furanone | biospider | | BHMDF | biospider | | dihydro-3,4-Bis((3-hydroxyphenyl)methyl)-2(3H)-furanone | HMDB | | Dihydro-3,4-bis(3-hydroxyphenyl)methyl-2(3H)-furanone, 9CI | db_source | | HPMF | db_source | | trans-2,3-Bis(3-hydroxybenzyl)-gamma-butyrolactone | biospider |
|
|---|
| Chemical Formula | C18H18O4 |
|---|
| IUPAC name | (3R,4R)-3,4-bis[(3-hydroxyphenyl)methyl]oxolan-2-one |
|---|
| InChI Identifier | InChI=1S/C18H18O4/c19-15-5-1-3-12(8-15)7-14-11-22-18(21)17(14)10-13-4-2-6-16(20)9-13/h1-6,8-9,14,17,19-20H,7,10-11H2/t14-,17+/m0/s1 |
|---|
| InChI Key | HVDGDHBAMCBBLR-WMLDXEAASA-N |
|---|
| Isomeric SMILES | OC1=CC=CC(C[C@H]2COC(=O)[C@@H]2CC2=CC(O)=CC=C2)=C1 |
|---|
| Average Molecular Weight | 298.3331 |
|---|
| Monoisotopic Molecular Weight | 298.120509064 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dibenzylbutyrolactone lignans. These are lignan compounds containing a 3,4-dibenzyloxolan-2-one moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Furanoid lignans |
|---|
| Sub Class | Tetrahydrofuran lignans |
|---|
| Direct Parent | Dibenzylbutyrolactone lignans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzylbutyrolactone
- Lignan lactone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Gamma butyrolactone
- Benzenoid
- Tetrahydrofuran
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | 1 to 2 mg |
|---|
| Delivery Time | 2 weeks |
|---|
| Storage Form | powder |
|---|
| Storage Conditions | -18°C |
|---|
| Stability | Not Available |
|---|
| Purity | unknown |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
| Contact Name | Contact Institution | Contact Email |
|---|
| Augustin Scalbert | International Agency for Research on Cancer (IARC), Biomarkers Group, 150 cours Albert Thomas, Lyon, FR, 69372 | scalberta@iarc.fr |
|
| Commercial Vendors |
|---|
| Not Available |