| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2018-05-02 12:13:02 UTC |
|---|
| Update date | 2018-05-04 14:23:50 UTC |
|---|
| FoodComEx ID | PC001183 |
|---|
| FoodDB Record | FDB021828 |
|---|
| Chemical Information |
|---|
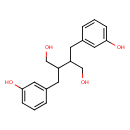
| Name | Enterodiol |
|---|
| Description | Enterodiol is one of the most important lignan-type phytoestrogens identified in serum, urine, bile and seminal fluids of humans and animals. Phytoestrogens are a diverse group of compounds found in many edible plants that have, as their common denominator, a phenolic group that they share with estrogenic steroids. This phenolic group appears to play an important role in determining the estrogenic agonist/antagonistic properties of these compounds. Phytoestrogens have been categorized according to their chemical structures as isoflavones, lignans and coumestans. Enterodiol is formed by bacteria in the intestinal tract from the plant lignans matairesinol and secoisolariciresinol, which exist in various whole-grain cereals (barley, rye and wheat), seeds, nuts, legumes and vegetables. (PMID: 12270221, J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Sep 25;777(1-2):289-309.) [HMDB]. Enterodiol is a biomarker for the consumption of soy beans and other soy products.
|
|---|
| CAS Number | 80226-00-2 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-Enterodiol | HMDB | | (2R,3R)-2,3-Bis[(3-hydroxyphenyl)methyl]-1,4-butanediol | HMDB | | [R-(R*,r*)]-2,3-bis[(3-hydroxyphenyl)methyl]-1,4-butanediol | HMDB | | Arbo 9 | HMDB |
|
|---|
| Chemical Formula | C18H22O4 |
|---|
| IUPAC name | 2,3-bis[(3-hydroxyphenyl)methyl]butane-1,4-diol |
|---|
| InChI Identifier | InChI=1S/C18H22O4/c19-11-15(7-13-3-1-5-17(21)9-13)16(12-20)8-14-4-2-6-18(22)10-14/h1-6,9-10,15-16,19-22H,7-8,11-12H2 |
|---|
| InChI Key | DWONJCNDULPHLV-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OCC(CC1=CC(O)=CC=C1)C(CO)CC1=CC=CC(O)=C1 |
|---|
| Average Molecular Weight | 302.3649 |
|---|
| Monoisotopic Molecular Weight | 302.151809192 |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | 1 to 2 mg |
|---|
| Delivery Time | 2 weeks |
|---|
| Storage Form | powder |
|---|
| Storage Conditions | 4°C |
|---|
| Stability | Not Available |
|---|
| Purity | unknown |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
| Contact Name | Contact Institution | Contact Email |
|---|
| Augustin Scalbert | International Agency for Research on Cancer (IARC), Biomarkers Group, 150 cours Albert Thomas, Lyon, FR, 69372 | scalberta@iarc.fr |
|
| Commercial Vendors |
|---|
| AKSci | 9828AL |
|---|