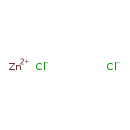| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:42 UTC |
|---|
| Update date | 2017-01-19 02:36:44 UTC |
|---|
| FoodComEx ID | PC001000 |
|---|
| FoodDB Record | FDB013485 |
|---|
| Chemical Information |
|---|
| Name | Zinc chloride |
|---|
| Description | Nutrient supplement
A number of salts containing the tetrachlorozincate anion, ZnCl2?4, are known. "Caulton's reagent," V2Cl3(thf)6Zn2Cl6 is an example of a salt containing Zn2Cl2?6. The compound Cs3ZnCl5 contains tetrahedral ZnCl2?4 and Cl? anions. No compounds containing the ZnCl4?6 ion have been characterized.; Four crystalline forms, (polymorphs) , of ZnCl2 are known, and in each case the Zn2+ ions are trigonal planar coordinated to four chloride ions. The pure anhydrous orthorhombic form rapidly changes to one of the other forms on exposure to the atmosphere and a possible explanation is that the presence of OH? facilitates the rearrangement. Rapid cooling of molten ZnCl2 gives a glass, that is, a rigid amorphous solid and this ability has been related to the structure in the melt. |
|---|
| CAS Number | 7646-85-7 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Butter of zinc | biospider | | Chlorure de zinc | biospider | | Dichlorozinc | biospider | | Hexite | biospider | | Hydrochloric acid zinc salt (2:1) | biospider | | Tinning flux | biospider | | Zinc (chlorure de) | biospider | | Zinc butter | biospider | | Zinc chloride (JP15/USP) | biospider | | Zinc chloride (TN) | biospider | | Zinc chloride (ZnCl2) | biospider | | Zinc chloride [usan:jan] | biospider | | Zinc chloride fume | biospider | | zinc chloride, (65)Zn-labeled | biospider | | Zinc chloride, anhydrous | biospider | | Zinc chloride, anhydrous [UN2331] [Corrosive] | biospider | | Zinc dichloride | biospider | | Zinc muriate | biospider | | Zinc(II) chloride | biospider | | Zinco | biospider | | Zinco (cloruro di) | biospider | | Zinctrace | db_source | | Zine dichloride | biospider | | Zinkchlorid | biospider | | Zinkchlorid (german) | biospider | | Zinkchloride | biospider | | Zintrace | biospider | | ZnCl2 | biospider |
|
|---|
| Chemical Formula | Cl2Zn |
|---|
| IUPAC name | zinc(2+) ion dichloride |
|---|
| InChI Identifier | InChI=1S/2ClH.Zn/h2*1H;/q;;+2/p-2 |
|---|
| InChI Key | JIAARYAFYJHUJI-UHFFFAOYSA-L |
|---|
| Isomeric SMILES | [Cl-].[Cl-].[Zn++] |
|---|
| Average Molecular Weight | 136.315 |
|---|
| Monoisotopic Molecular Weight | 133.866851992 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of inorganic compounds known as transition metal chlorides. These are inorganic compounds in which the largest halogen atom is Chlorine, and the heaviest metal atom is a transition metal. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Transition metal salts |
|---|
| Sub Class | Transition metal chlorides |
|---|
| Direct Parent | Transition metal chlorides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Transition metal chloride
- Inorganic chloride salt
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 318° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | Not Available |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | R999 |
|---|
| Glentham | GK8554 |
|---|
| Toronto Research Chemicals | Z438900 |
|---|