| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:35 UTC |
|---|
| Update date | 2017-01-19 02:36:42 UTC |
|---|
| FoodComEx ID | PC000954 |
|---|
| FoodDB Record | FDB008735 |
|---|
| Chemical Information |
|---|
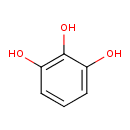
| Name | 1,2,3-Benzenetriol |
|---|
| Description | 1,2,3-trihydroxybenzene, also known as pyrogallic acid or 1,2,3-benzenetriol, is a member of the class of compounds known as 5-unsubstituted pyrrogallols. 5-unsubstituted pyrrogallols are pyrrogallols that are unsubstituted at th5-position of the benzene ring. 1,2,3-trihydroxybenzene is soluble (in water) and a very weakly acidic compound (based on its pKa). 1,2,3-trihydroxybenzene can be found in arabica coffee, beer, cocoa powder, and coffee, which makes 1,2,3-trihydroxybenzene a potential biomarker for the consumption of these food products. 1,2,3-trihydroxybenzene can be found primarily in blood, feces, and urine. 1,2,3-trihydroxybenzene is an organic compound with the formula C6H3(OH)3. It is a white water-soluble solid although samples are typically brownish because of its sensitivity toward oxygen. It is one of three isomeric benzenetriols . |
|---|
| CAS Number | 87-66-1 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1,2,3-Trihydroxybenzene | db_source | | Pyrogallic acid | db_source | | Pyrogallol | manual | | Pyrogallol, 8CI | db_source |
|
|---|
| Chemical Formula | C6H6O3 |
|---|
| IUPAC name | benzene-1,2,3-triol |
|---|
| InChI Identifier | InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H |
|---|
| InChI Key | WQGWDDDVZFFDIG-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | [H]OC1=CC=CC(O[H])=C1O[H] |
|---|
| Average Molecular Weight | 126.11 |
|---|
| Monoisotopic Molecular Weight | 126.031694058 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 5-unsubstituted pyrrogallols. These are pyrrogallols that are unsubstituted at th5-position of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Benzenetriols and derivatives |
|---|
| Direct Parent | 5-unsubstituted pyrrogallols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 5-unsubstituted pyrrogallol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Polyol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 3 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | K567 |
|---|
| Cayman Chemical | 20347 |
|---|
| Glentham | GE8401 |
|---|
| MetaSci | HMDB0013674 |
|---|
| Sigma-Aldrich | HMDB0013674 |
|---|
| Toronto Research Chemicals | P997430 |
|---|