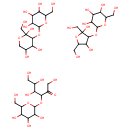| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:29 UTC |
|---|
| Update date | 2017-01-19 02:36:42 UTC |
|---|
| FoodComEx ID | PC000939 |
|---|
| FoodDB Record | FDB002107 |
|---|
| Chemical Information |
|---|
| Name | Turanose |
|---|
| Description | Isolated from honey
D-(+)-Turanose is a reducing disaccharide. Its systematic name is a-D-glucopyranosyl-(1-->3)-a-D-fructofuranose. It is an analog of sucrose not metabolized by higher plants, but rather acquired through the action of sucrose transporters for intracellular carbohydrate signaling. In addition to its involvement in signal transduction, D-(+)-Turanose can also be used as a carbon source by many organisms including numerous species of bacteria and fungi.; ; From Wiki; Turanose is a reducing disaccharide. The D-isomer is naturally occuring. Its systematic name is ?-D-glucopyranosyl-(1?3)-?-D-fructofuranose. It is an analog of sucrose not metabolized by higher plants, but rather acquired through the action of sucrose transporters for intracellular carbohydrate signaling. In addition to its involvement in signal transduction, D-(+)-turanose can also be used as a carbon source by many organisms including numerous species of bacteria and fungi. |
|---|
| CAS Number | 547-25-1 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 3-O-a-D-Glucopyranosyl-D-fructose | db_source | | 3-O-alpha-D-glucopyranosyl-D-Fructose | biospider | | 3-O-hexopyranosylhex-2-ulose | biospider | | 3-O-α-D-glucopyranosyl-D-fructose | Generator | | a-D-Glcp-(1->3)-D-fru | Generator | | alpha-D-Glcp-(1->3)-D-Fru | biospider | | alpha-D-glucopyranosyl-(1->3)-D-fructose | biospider | | D-(+)-turanose | biospider | | D-(+)-turanose (van) | biospider | | D-Fructose, 3-O-α-D-glucopyranosyl- | biospider | | D-Fructose, 3-O-alpha-D-glucopyranosyl- | biospider | | D-turanose | biospider | | Turanose (van) | biospider | | Turanose (VAN) (8CI) | biospider | | α-D-glcp-(1->3)-D-fru | Generator |
|
|---|
| Chemical Formula | C36H66O33 |
|---|
| IUPAC name | 1,4,5,6-tetrahydroxy-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}hexan-2-one; 2-(hydroxymethyl)-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxane-2,4,5-triol; 2-{[2,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-3-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| InChI Identifier | InChI=1S/3C12H22O11/c13-1-5-7(17)8(18)9(19)11(22-5)23-10-6(16)4(15)2-21-12(10,20)3-14;13-1-4-6(16)8(18)9(19)11(21-4)22-10-7(17)5(2-14)23-12(10,20)3-15;13-1-4(16)7(18)11(5(17)2-14)23-12-10(21)9(20)8(19)6(3-15)22-12/h2*4-11,13-20H,1-3H2;4,6-16,18-21H,1-3H2 |
|---|
| InChI Key | DTUQSKSQTMCUHD-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OCC(O)C(O)C(OC1OC(CO)C(O)C(O)C1O)C(=O)CO.OCC1OC(O)(CO)C(OC2OC(CO)C(O)C(O)C2O)C1O.OCC1OC(OC2C(O)C(O)COC2(O)CO)C(O)C(O)C1O |
|---|
| Average Molecular Weight | 1026.8894 |
|---|
| Monoisotopic Molecular Weight | 1026.348634638 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl glycosides |
|---|
| Direct Parent | Fatty acyl glycosides of mono- and disaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- C-glycosyl compound
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Fatty alcohol
- Beta-hydroxy ketone
- Oxane
- Alpha-hydroxy ketone
- Tetrahydrofuran
- Secondary alcohol
- Hemiacetal
- Ketone
- Polyol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Primary alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Alcohol
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -4.80 | MEYLAN,WM & HOWARD,PH (1995) |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 157° (168°) | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 7543AL |
|---|
| MetaSci | HMDB0011740 |
|---|
| Tokyo Chemical Industry | HMDB0011740 |
|---|
| Toronto Research Chemicals | G423500 |
|---|