| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:28 UTC |
|---|
| Update date | 2017-01-19 02:36:41 UTC |
|---|
| FoodComEx ID | PC000935 |
|---|
| FoodDB Record | FDB028791 |
|---|
| Chemical Information |
|---|
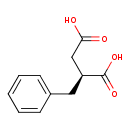
| Name | (R)-2-Benzylsuccinate |
|---|
| Description | (R)-2-Benzylsuccinate is an aromatic compounds that is an intermediate in Benzoate degradation via CoA ligation. Biodegradation of aromatic compounds is a common process in anoxic environments. The many natural and synthetic aromatic compounds found in the environment are usually degraded by anaerobic microorganisms into only few central intermediates, prior to ring cleavage. Benzoyl-CoA is the most important of these intermediates since a large number of compounds, including chloro-, nitro-, and aminobenzoates, aromatic hydrocarbons, and phenolic compounds, are initially converted to benzoyl-CoA prior to ring reduction and cleavage. (R)-2-Benzylsuccinate can be generated from toluene via the enzyme benzylsuccinate synthase (EC 4.1.99.11). It is then converted to Benzylsuccinyl-CoA via the enzyme benzylsuccinate CoA-transferase BbsE subunit (EC 2.8.3.15). [HMDB] |
|---|
| CAS Number | 884-33-3 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2R)-2-benzylsuccinic acid | hmdb | | (R)-2-Benzylsuccinic acid | hmdb | | L-benzylsuccinate | hmdb | | L-benzylsuccinic acid | hmdb |
|
|---|
| Chemical Formula | C11H12O4 |
|---|
| IUPAC name | (2S)-2-benzylbutanedioic acid |
|---|
| InChI Identifier | InChI=1S/C11H12O4/c12-10(13)7-9(11(14)15)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,12,13)(H,14,15)/t9-/m0/s1 |
|---|
| InChI Key | GTOFKXZQQDSVFH-VIFPVBQESA-N |
|---|
| Isomeric SMILES | OC(=O)C[C@H](CC1=CC=CC=C1)C(O)=O |
|---|
| Average Molecular Weight | 208.2106 |
|---|
| Monoisotopic Molecular Weight | 208.073558872 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as phenylpropanoic acids. Phenylpropanoic acids are compounds with a structure containing a benzene ring conjugated to a propanoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Phenylpropanoic acids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenylpropanoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-phenylpropanoic-acid
- Benzenoid
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 100 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 4543AB |
|---|
| MetaSci | HMDB0012127 |
|---|
| Sigma-Aldrich | HMDB0012127 |
|---|
| Toronto Research Chemicals | B292000 |
|---|