| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:28 UTC |
|---|
| Update date | 2017-01-19 02:36:41 UTC |
|---|
| FoodComEx ID | PC000934 |
|---|
| FoodDB Record | FDB022040 |
|---|
| Chemical Information |
|---|
| Name | 3-Hydroxyglutaric acid |
|---|
| Description | 3-Hydroxyglutaric acid is a key metabolite in glutaryl co-enzyme A dehydrogenase deficiency, and is considered to be a potential neurotoxin. The urine level of 3-Hydroxyglutaric acid is elevated in Glutaric Aciduria Type I (glutaryl-CoA dehydrogenase deficiency) patients. (PMID 16573641) [HMDB] |
|---|
| CAS Number | 638-18-6 |
|---|
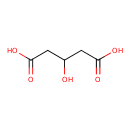
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 3-hydroxy-glutarate | hmdb | | 3-hydroxy-glutaric acid | hmdb | | 3-Hydroxyglutarate | hmdb | | 3-Hydroxyglutaric acid | hmdb |
|
|---|
| Chemical Formula | C5H8O5 |
|---|
| IUPAC name | 3-hydroxypentanedioic acid |
|---|
| InChI Identifier | InChI=1S/C5H8O5/c6-3(1-4(7)8)2-5(9)10/h3,6H,1-2H2,(H,7,8)(H,9,10) |
|---|
| InChI Key | ZQHYXNSQOIDNTL-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(CC(O)=O)CC(O)=O |
|---|
| Average Molecular Weight | 148.114 |
|---|
| Monoisotopic Molecular Weight | 148.037173366 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Short-chain hydroxy acid
- Beta-hydroxy acid
- Fatty acid
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Cayman Chemical | 16334 |
|---|
| Toronto Research Chemicals | H942580 |
|---|