| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:26 UTC |
|---|
| Update date | 2017-01-19 02:36:41 UTC |
|---|
| FoodComEx ID | PC000922 |
|---|
| FoodDB Record | FDB022788 |
|---|
| Chemical Information |
|---|
| Name | 5-Methoxydimethyltryptamine |
|---|
| Description | 5-Methoxydimethyltryptamine, like all Methoxydimethyltryptamines is a compound that contain the biogenic monoamine tryptamine and is substituted with one methoxy group and two methyl groups. Members of this group include several potent serotonergic hallucinogens found in several unrelated plants, skins of certain toads, and in mammalian brains. They are possibly involved in the etiology of schizophrenia. (PubChem)
They are formed as metabolites of serotonin (5-hydroxytryptamine) or tryptamine by the enzyme indolethylamine N-methyltransferase (INMT). The physiological significance of the N-methylating pathway of indoleamine metabolism, and of the methylated end products, is unknown. Because of the known psychotropic properties of the dimethylated amines, their possible involvement in the chemical pathogenesis of mental disorders has received wide interest. The hallucinogenic actions of the methylated indoleamines, like those of LSD, are believed to be mediated through the 5HT2 receptor. (PMID 11763413) [HMDB] |
|---|
| CAS Number | 1019-45-0 |
|---|
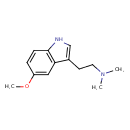
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 3-(2-(N,N-Dimethyl)aminoethyl)-5-methoxyindole | hmdb | | 3-(2-Dimethylaminoethyl)-5-methoxyindole | ChEBI | | 3-[2-(N,N-dimethylamino)Ethyl]-5-methoxy-indole | ChEBI | | 5-MeO-dmt | ChEBI | | 5-methoxy-N,N-dimethyl-1H-Indole-3-ethanamine | hmdb | | 5-Methoxy-N,N-dimethyl-1H-indole-3-ethylamine | hmdb | | 5-Methoxy-N,N-dimethyltryptamine | hmdb | | 5-methoxyindole 3-(2-(N,N-Dimethylamino)ethyl) | hmdb | | Bufotenine, 5-Methoxydimethyltryptamine | hmdb | | MeODMT | ChEBI | | Methoxybufotenin | hmdb | | Methylbufotenine | hmdb | | N,N-Dimethyl-5-methoxy tryptamine | hmdb | | N,N-Dimethyl-5-methoxytryptamine | ChEBI | | O-methylbufotenine | hmdb |
|
|---|
| Chemical Formula | C13H18N2O |
|---|
| IUPAC name | [2-(5-methoxy-1H-indol-3-yl)ethyl]dimethylamine |
|---|
| InChI Identifier | InChI=1S/C13H18N2O/c1-15(2)7-6-10-9-14-13-5-4-11(16-3)8-12(10)13/h4-5,8-9,14H,6-7H2,1-3H3 |
|---|
| InChI Key | ZSTKHSQDNIGFLM-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | COC1=CC2=C(NC=C2CCN(C)C)C=C1 |
|---|
| Average Molecular Weight | 218.2948 |
|---|
| Monoisotopic Molecular Weight | 218.141913208 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as tryptamines and derivatives. Tryptamines and derivatives are compounds containing the tryptamine backbone, which is structurally characterized by an indole ring substituted at the 3-position by an ethanamine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Tryptamines and derivatives |
|---|
| Direct Parent | Tryptamines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tryptamine
- 3-alkylindole
- Indole
- Alkaloid or derivatives
- Anisole
- Alkyl aryl ether
- Aralkylamine
- Benzenoid
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Ether
- Organooxygen compound
- Organonitrogen compound
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 20 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | M269805 |
|---|