| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:19 UTC |
|---|
| Update date | 2017-01-19 02:36:40 UTC |
|---|
| FoodComEx ID | PC000907 |
|---|
| FoodDB Record | FDB022498 |
|---|
| Chemical Information |
|---|
| Name | Prostaglandin E2 |
|---|
| Description | Dinoprostone is a naturally occurring prostaglandin E2 (PGE2) and the most common and most biologically active of the mammalian prostaglandins. It has important effects in labour and also stimulates osteoblasts to release factors which stimulate bone resorption by osteoclasts (a type of bone cell that removes bone tissue by removing the bone's mineralized matrix). PGE2 has been shown to increase vasodilation and cAMP production, to enhance the effects of bradykinin and histamine, induction of uterine contractions and of platelet aggregation. PGE2 is also responsible for maintaining the open passageway of the fetal ductus arteriosus; decreasing T-cell proliferation and lymphocyte migration and activating the secretion of IL-1α and IL-2. PGE2 exhibits both pro- and anti-inflammatory effects, particularly on dendritic cells (DC). Depending on the nature of maturation signals, PGE2 has different and sometimes opposite effects on DC biology. PGE2 exerts an inhibitory action, reducing the maturation of DC and their ability to present antigen. PGE2 has also been shown to stimulate DC and promote IL-12 production when given in combination with TNF-alpha. PGE2 is an environmentally bioactive substance. Its action is prolonged and sustained by other factors especially IL-10. It modulates the activities of professional DC by acting on their differentiation, maturation and their ability to secrete cytokines. PGE2 is a potent inducer of IL-10 in bone marrow-derived DC (BM-DC), and PGE2-induced IL-10 is a key regulator of the BM-DC pro-inflammatory phenotype. (PMID: 16978535)
Dinoprostone is a naturally occurring prostaglandin E2 (PGE2) and the most common and most biologically active of the mammalian prostaglandins. It has important effects in labour and also stimulates osteoblasts to release factors which stimulate bone resorption by osteoclasts (a type of bone cell that removes bone tissue by removing the bone's mineralized matrix). PGE2 has been shown to increase vasodilation and cAMP production, to enhance the effects of bradykinin and histamine, to induce uterine contractions and to activate platelet aggregation. PGE2 is also responsible for maintaining the open passageway of the fetal ductus arteriosus; decreasing T-cell proliferation and lymphocyte migration and activating the secretion of IL-1alpha and IL-2. PGE2 exhibits both pro- and anti-inflammatory effects, particularly on dendritic cells (DC). Depending on the nature of maturation signals, PGE2 has different and sometimes opposite effects on DC biology. PGE2 exerts an inhibitory action, reducing the maturation of DC and their ability to present antigen. PGE2 has also been shown to stimulate DC and promote IL-12 production when given in combination with TNF-alpha. PGE2 is an environmentally bioactive substance. Its action is prolonged and sustained by other factors especially IL-10. It modulates the activities of professional DC by acting on their differentiation, maturation and their ability to secrete cytokines. PGE2 is a potent inducer of IL-10 in bone marrow-derived DC (BM-DC), and PGE2-induced IL-10 is a key regulator of the BM-DC pro-inflammatory phenotype. (PMID: 16978535)
Prostaglandins are eicosanoids. The eicosanoids consist of the prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs) and lipoxins (LXs). The PGs and TXs are collectively identified as prostanoids. Prostaglandins were originally shown to be synthesized in the prostate gland, thromboxanes from platelets (thrombocytes) and leukotrienes from leukocytes, hence the derivation of their names. All mammalian cells except erythrocytes synthesize eicosanoids. These molecules are extremely potent, able to cause profound physiological effects at very dilute concentrations. All eicosanoids function locally at the site of synthesis, through receptor-mediated G-protein linked signaling pathways. [HMDB]. Prostaglandin E2 is found in many foods, some of which are common sage, nanking cherry, colorado pinyon, and sweet basil. |
|---|
| CAS Number | 363-24-6 |
|---|
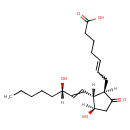
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-Prostaglandin E2 | hmdb | | (15S)-Prostaglandin e2 | ChEBI | | (5Z,11a,13e,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-Oate | Generator | | (5Z,11a,13e,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-Oic acid | Generator | | (5Z,11alpha,13e,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-Oate | Generator | | (5Z,11alpha,13e,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-Oic acid | ChEBI | | (5Z,11α,13e,15S)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-Oate | Generator | | (5Z,11α,13e,15S)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-Oic acid | Generator | | (5Z,13E,15S)-11-alpha,15-dihydroxy-9-oxoprost-5,13-dienoate | hmdb | | (5Z,13E,15S)-11-alpha,15-dihydroxy-9-oxoprost-5,13-dienoic acid | hmdb | | (5Z,13e)-(15S)-11a,15-Dihydroxy-9-oxoprost-13-enoate | Generator | | (5Z,13e)-(15S)-11a,15-Dihydroxy-9-oxoprost-13-enoic acid | Generator | | (5Z,13e)-(15S)-11a,15-Dihydroxy-9-oxoprosta-5,13-dienoate | Generator | | (5Z,13e)-(15S)-11a,15-Dihydroxy-9-oxoprosta-5,13-dienoic acid | Generator | | (5Z,13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprost-13-enoate | hmdb | | (5Z,13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprost-13-enoic acid | hmdb | | (5Z,13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprosta-5,13-dienoate | hmdb | | (5Z,13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprosta-5,13-dienoic acid | hmdb | | (5Z,13e)-(15S)-11α,15-dihydroxy-9-oxoprost-13-enoate | Generator | | (5Z,13e)-(15S)-11α,15-dihydroxy-9-oxoprost-13-enoic acid | Generator | | (5Z,13e)-(15S)-11α,15-dihydroxy-9-oxoprosta-5,13-dienoate | Generator | | (5Z,13e)-(15S)-11α,15-dihydroxy-9-oxoprosta-5,13-dienoic acid | Generator | | (e,Z)-(1R,2R,3R)-7-(3-Hydroxy-2-((3S)-(3-hydroxy-1-octenyl))-5-oxocyclopentyl)-5-heptenoate | Generator | | (e,Z)-(1R,2R,3R)-7-(3-Hydroxy-2-((3S)-(3-hydroxy-1-octenyl))-5-oxocyclopentyl)-5-heptenoic acid | ChEBI | | (Z)-7-((1R,2R,3R)-3-Hydroxy-2-((S,e)-3-hydroxyoct-1-enyl)-5-oxocyclopentyl)hept-5-enoate | Generator | | (Z)-7-((1R,2R,3R)-3-Hydroxy-2-((S,e)-3-hydroxyoct-1-enyl)-5-oxocyclopentyl)hept-5-enoic acid | ChEBI | | 5-trans-PGE2 | hmdb | | Dinoproston | ChEBI | | Dinoprostona | ChEBI | | Dinoprostone | hmdb | | Dinoprostonum | hmdb | | Glandin | hmdb | | l-Prostaglandin E2 | hmdb | | Minprositin E2 | hmdb | | Minprostin E2 | hmdb | | PGE2 | hmdb | | Prepidil | hmdb | | Propess | ChEBI | | Prostaglandin E | hmdb | | Prostaglandin E2 | hmdb | | Prostaglandin E2alpha | hmdb | | Prostarmon E | hmdb | | Prostin | hmdb | | Prostin E2 | hmdb |
|
|---|
| Chemical Formula | C20H32O5 |
|---|
| IUPAC name | 7-[(1R,2R,3R)-3-hydroxy-2-[(3S)-3-hydroxyoct-1-en-1-yl]-5-oxocyclopentyl]hept-5-enoic acid |
|---|
| InChI Identifier | InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/t15-,16+,17+,19+/m0/s1 |
|---|
| InChI Key | XEYBRNLFEZDVAW-DODZYUBVSA-N |
|---|
| Isomeric SMILES | [H]C(=C[C@@]1([H])[C@]([H])(O)CC(=O)[C@]1([H])CC=CCCCC(O)=O)[C@@]([H])(O)CCCCC |
|---|
| Average Molecular Weight | 352.4651 |
|---|
| Monoisotopic Molecular Weight | 352.224974134 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Prostaglandins and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Prostaglandin skeleton
- Long-chain fatty acid
- Hydroxy fatty acid
- Cyclopentanol
- Fatty acid
- Unsaturated fatty acid
- Cyclic alcohol
- Ketone
- Cyclic ketone
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | E847 |
|---|
| Cayman Chemical | 10007211 |
|---|
| Cayman Chemical | 14010 |
|---|
| Toronto Research Chemicals | P838610 |
|---|