| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:19 UTC |
|---|
| Update date | 2017-01-19 02:36:40 UTC |
|---|
| FoodComEx ID | PC000905 |
|---|
| FoodDB Record | FDB023096 |
|---|
| Chemical Information |
|---|
| Name | Canrenone |
|---|
| Description | Canrenone is the major metabolite of spironolactone. Spironolactone is a competitive aldosterone receptor antagonist (ARA), has traditionally been the treatment of first choice in idiopathic hyperaldosteronism (IHA) and for preoperative management of aldosterone producing adenoma (APA), and its therapeutic properties are attributable to active metabolite canrenone. Canrenone and the K+ salt of canrenoate are also in clinical use: they avoid the formation of intermediate products with anti-androgenic and progestational actions, resulting in a decreased incidence of side effects. (PMID: 10790593) [HMDB] |
|---|
| CAS Number | 976-71-6 |
|---|
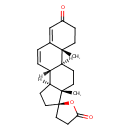
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 17-Hydroxy-3-oxo-17a-pregna-4,6-diene-21-carboxylic acid g-lactone | hmdb | | 17-Hydroxy-3-oxo-17a-pregna-4,6-diene-21-carboxylic acid lactone | hmdb | | 17a-(2-Carboxyethyl)-17b-hydroxyandrosta-4,6-dien-3-one lactone | hmdb | | 17b-Hydroxy-3-oxopregna-4,6-diene-21-carboxylic acid | hmdb | | 20-Spiroxa-4,6-diene-3,21-dione | hmdb | | 3-(17b-Hydroxy-3-oxoandrosta-4,6-dien-17a-yl)propionic acid g-lactone | hmdb | | 3-(17b-Hydroxy-3-oxoandrosta-4,6-dien-17a-yl)propionic acid lactone | hmdb | | 3-(3-Oxo-17b-hydroxy-4,6-androstadien-17a-yl)propionic acid g-lactone | hmdb | | 3'-(3-Oxo-17b-hydroxyandrosta-4,6-dien-17a-yl)-propionic acid lactone | hmdb | | Aldadiene | hmdb | | Canrenone | hmdb | | Phanurane | hmdb |
|
|---|
| Chemical Formula | C22H28O3 |
|---|
| IUPAC name | (1'S,2R,2'R,10'R,11'S,15'S)-2',15'-dimethylspiro[oxolane-2,14'-tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecane]-6',8'-diene-5,5'-dione |
|---|
| InChI Identifier | InChI=1S/C22H28O3/c1-20-9-5-15(23)13-14(20)3-4-16-17(20)6-10-21(2)18(16)7-11-22(21)12-8-19(24)25-22/h3-4,13,16-18H,5-12H2,1-2H3/t16-,17+,18+,20+,21+,22-/m1/s1 |
|---|
| InChI Key | UJVLDDZCTMKXJK-WNHSNXHDSA-N |
|---|
| Isomeric SMILES | [H][C@@]12CC[C@@]3(CCC(=O)O3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=CC2=CC(=O)CC[C@]12C |
|---|
| Average Molecular Weight | 340.4559 |
|---|
| Monoisotopic Molecular Weight | 340.203844762 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as steroid lactones. These are sterol lipids containing a lactone moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid lactones |
|---|
| Direct Parent | Steroid lactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid lactone
- 3-oxosteroid
- Oxosteroid
- Cyclohexenone
- Gamma butyrolactone
- Tetrahydrofuran
- Cyclic ketone
- Lactone
- Ketone
- Carboxylic acid ester
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Oxacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H489 |
|---|
| AKSci | J10438 |
|---|
| AKSci | J40163 |
|---|
| AKSci | J91753 |
|---|
| AKSci | HMDB0003033 |
|---|
| MetaSci | HMDB0003033 |
|---|
| Toronto Research Chemicals | C175610 |
|---|