| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:18 UTC |
|---|
| Update date | 2017-01-19 02:36:40 UTC |
|---|
| FoodComEx ID | PC000900 |
|---|
| FoodDB Record | FDB012521 |
|---|
| Chemical Information |
|---|
| Name | Campesterol |
|---|
| Description | Constituent of rapeseed oil (Brassica napa), soybean oil (Glycine max) and wheatgerm oil (Triticum subspecies). Found in virtually all plant oils
Campesterol is a phytosterol, meaning it is a steroid derived from plants. As a food additive, phytosterols have cholesterol-lowering properties (reducing cholesterol absorption in intestines), and may act in cancer prevention. Phytosterols naturally occur in small amount in vegetable oils, especially soybean oil. One such phytosterol complex, isolated from vegetable oil, is cholestatin, composed of campesterol, stigmasterol, and brassicasterol, and is marketed as a dietary supplement. Sterols can reduce cholesterol in human subjects by up to 15%.; ; The mechanism behind phytosterols and the lowering of cholesterol occurs as follows : the incorporation of cholesterol into micelles in the gastrointestinal tract is inhibited, decreasing the overall amount of cholesterol absorbed. This may in turn help to control body total cholesterol levels, as well as modify HDL, LDL and TAG levels. Many margarines, butters, breakfast cereals and spreads are now enriched with phytosterols and marketed towards people with high cholesterol and a wish to lower it. -- Wikipedia; Presence of brassicasterol, together with auxiliary markers ?-linolenic acid and erucic acid, is a marker of adulteration of soybean oil and sunflower oil with rapeseed oil. As there is no brassicasterol in sunflower and soybean oil, but its concentration in rapeseed oil is about 1400 mg/kg, the amount of rapeseed oil added can be calculated. |
|---|
| CAS Number | 474-62-4 |
|---|
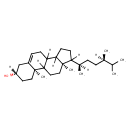
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (24R)-5-Ergosten-3-beta-ol | biospider | | (24R)-5-Ergosten-3α-Methylcholesterol | biospider | | (24R)-5-Ergosten-3β-ol | biospider | | (24R)-5-Ergosten-3b-ol | biospider | | (24R)-Ergost-5-en-3-beta-ol | biospider | | (24R)-Ergost-5-en-3b-ol | biospider | | (24R)-Methylcholest-5-en-3β-ol | biospider | | (24R)-Methylcholest-5-en-3b-ol | biospider | | (3-beta-24R)-Ergost-5-en-3-ol | biospider | | (3-beta)-Ergost-5-en-3-ol | biospider | | (3b,24R)-Ergost-5-en-3-ol | biospider | | (3b)-Ergost-5-en-3-ol | Generator | | (3beta)-Ergost-5-en-3-ol | ChEBI | | (3β)-ergost-5-en-3-ol | Generator | | «DELTA»5-24-Isoergosten-3β-ol | biospider | | 24-alpha-Methylcholesterol | biospider | | 24-methyl-5-Cholestene-3-ol | biospider | | 24-Methylcholest-5-en-3beta-ol | biospider | | 24α-Methyl-5-cholesten-3β-ol | biospider | | 24α-Methylcholesterol | biospider | | 24a-Methyl-5-cholesten-3b-ol | biospider | | 24a-Methylcholesterol | biospider | | 5-Cholestene-3-ol, 24-methyl- | biospider | | Campesterin | biospider | | Campesterol | db_source | | Campestrol | biospider | | EB 82A | db_source | | Ergost-5-en-3-beta-ol | biospider | | Ergost-5-en-3-ol | biospider | | Ergost-5-en-3-ol, (3β,24R)- | biospider | | Ergost-5-en-3β-ol, (24R)- | biospider | | Mieyajunsu A | db_source |
|
|---|
| Chemical Formula | C28H48O |
|---|
| IUPAC name | (2R,5S,15R)-14-[(2R,5R)-5,6-dimethylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol |
|---|
| InChI Identifier | InChI=1S/C28H48O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h9,18-20,22-26,29H,7-8,10-17H2,1-6H3/t19-,20-,22+,23?,24?,25?,26?,27+,28-/m1/s1 |
|---|
| InChI Key | SGNBVLSWZMBQTH-MMTVXVTESA-N |
|---|
| Isomeric SMILES | CC(C)[C@H](C)CC[C@@H](C)C1CCC2C3CC=C4C[C@@H](O)CC[C@]4(C)C3CC[C@]12C |
|---|
| Average Molecular Weight | 400.6801 |
|---|
| Monoisotopic Molecular Weight | 400.370516158 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as ergosterols and derivatives. These are steroids containing ergosta-5,7,22-trien-3beta-ol or a derivative thereof, which is based on the 3beta-hydroxylated ergostane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Ergostane steroids |
|---|
| Direct Parent | Ergosterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ergosterol-skeleton
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Hydroxysteroid
- 3-hydroxysteroid
- 3-hydroxy-delta-5-steroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 157-158° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |