| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:14 UTC |
|---|
| Update date | 2017-01-19 02:36:40 UTC |
|---|
| FoodComEx ID | PC000888 |
|---|
| FoodDB Record | FDB023340 |
|---|
| Chemical Information |
|---|
| Name | Pyrrole-2-carboxylic acid |
|---|
| Description | Pyrrole-2-carboxylic acid was synthesized over a century ago, but its history as a compound of biological origin is rather recent. It was first identified as a degradation product of sialic acids, then as a derivative of the oxidation of the D-hydroxyproline isomers by mammalian D-amino acid oxidase. The latter relationship results from the lability of the direct oxidation product, A'-pyrroline-4-hydroxy-2-carboxylic acid, which loses water spontaneously to form the pyrrole. A similar reaction is catalyzed by the more specific allohydroxy-D-proline oxidase of Pseudomonas. In whole animal observations, pyrrole-2-carboxylate (PCA) ' was identified in rat or human urine after administration of the D-isomers of hydroxyproline, a finding ascribable to the action of D-amino acid oxidase. (PMID: 4430715)
Urinary excretion of N-(pyrrole-2-carboxyl) glycine has been reported in a 5-year-old affected with type II hyperprolinemia; The child has mild developmental delay, recurrent seizures of the grand mal type and EEG alterations. The urinary excretion of the conjugate is stressed, since it appears that only one previous report in the literature described this compound in the urine of two patients affected by this disturbance. (PMID 2383933) [HMDB] |
|---|
| CAS Number | 634-97-9 |
|---|
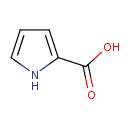
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1H-Pyrrole-2-carboxylic acid | hmdb | | 1H-Pyrrole-2-carboxylic acid (9CI) | hmdb | | 2-Minaline | hmdb | | 2-Pyrrolecarboxylate | Generator | | 2-Pyrrolecarboxylic acid | hmdb | | Minalin | hmdb | | Minaline | hmdb | | PCA | hmdb | | PYC | hmdb | | Pyrrole-2-carboxylate | hmdb | | Pyrrole-2-carboxylic acid | hmdb |
|
|---|
| Chemical Formula | C5H5NO2 |
|---|
| IUPAC name | 1H-pyrrole-2-carboxylic acid |
|---|
| InChI Identifier | InChI=1S/C5H5NO2/c7-5(8)4-2-1-3-6-4/h1-3,6H,(H,7,8) |
|---|
| InChI Key | WRHZVMBBRYBTKZ-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(=O)C1=CC=CN1 |
|---|
| Average Molecular Weight | 111.0987 |
|---|
| Monoisotopic Molecular Weight | 111.032028409 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrrole 2-carboxylic acids. These are pyrrole carboxylic acids where the carboxyl group is attached at position C2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrroles |
|---|
| Sub Class | Pyrrole carboxylic acids and derivatives |
|---|
| Direct Parent | Pyrrole 2-carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrole-2-carboxylic acid
- Substituted pyrrole
- Heteroaromatic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 300 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | J90828 |
|---|
| AKSci | Q622 |
|---|
| AKSci | HMDB0004230 |
|---|
| MetaSci | HMDB0004230 |
|---|
| Toronto Research Chemicals | P997890 |
|---|