| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:12 UTC |
|---|
| Update date | 2017-01-19 02:36:40 UTC |
|---|
| FoodComEx ID | PC000881 |
|---|
| FoodDB Record | FDB023194 |
|---|
| Chemical Information |
|---|
| Name | Salicin |
|---|
| Description | Salicin is an alcoholic β-glycoside that contains D-glucose. Salicin is an anti-inflammatory agent that is produced from willow bark. Salicin is closely related in chemical make-up to aspirin and has a very similar action in the human body. When consumed by humans, Salicin is metabolized into salicylic acid. [HMDB] |
|---|
| CAS Number | 138-52-3 |
|---|
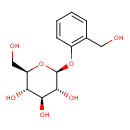
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-(Hydroxymethyl)phenyl hexopyranoside | hmdb | | 2-(Hydroxymethyl)phenyl-b-D-glucopyranoside | Generator | | 2-(Hydroxymethyl)phenyl-beta-D-glucopyranoside | ChEBI | | 2-(Hydroxymethyl)phenyl-O-b-D-glucopyranoside | Generator | | 2-(Hydroxymethyl)phenyl-O-beta-D-glucopyranoside | ChEBI | | 2-(Hydroxymethyl)phenyl-O-β-D-glucopyranoside | Generator | | 2-(Hydroxymethyl)phenyl-β-D-glucopyranoside | Generator | | 2(Hydroxymethyl)phenyl-b-D-glucopyranoside | Generator | | 2(Hydroxymethyl)phenyl-beta-D-glucopyranoside | ChEBI | | 2(Hydroxymethyl)phenyl-β-D-glucopyranoside | Generator | | D-(-)-Salicin | ChEBI | | D-Salicin | hmdb | | delta-Salicin | hmdb | | O-(Hydroxymethyl)phenyl b-D-glucopyranoside | Generator | | O-(Hydroxymethyl)phenyl beta-D-glucopyranoside | ChEBI | | O-(Hydroxymethyl)phenyl β-D-glucopyranoside | Generator | | Salicin | hmdb | | Salicine | hmdb | | Salicoside | hmdb | | Salicyl alcohol glucoside | hmdb | | Saligenin b-D-glucopyranoside | Generator | | Saligenin beta-D-glucopyranoside | hmdb | | Saligenin beta-delta-glucopyranoside | hmdb | | Saligenin β-D-glucopyranoside | Generator | | Saligenin-b-D-glucopyranoside | hmdb | | Saligenin-beta-D-glucopyranoside | hmdb | | Saligenin-beta-delta-glucopyranoside | hmdb |
|
|---|
| Chemical Formula | C13H18O7 |
|---|
| IUPAC name | (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[2-(hydroxymethyl)phenoxy]oxane-3,4,5-triol |
|---|
| InChI Identifier | InChI=1S/C13H18O7/c14-5-7-3-1-2-4-8(7)19-13-12(18)11(17)10(16)9(6-15)20-13/h1-4,9-18H,5-6H2/t9-,10-,11+,12-,13-/m1/s1 |
|---|
| InChI Key | NGFMICBWJRZIBI-UJPOAAIJSA-N |
|---|
| Isomeric SMILES | OC[C@H]1O[C@@H](OC2=C(CO)C=CC=C2)[C@H](O)[C@@H](O)[C@@H]1O |
|---|
| Average Molecular Weight | 286.2778 |
|---|
| Monoisotopic Molecular Weight | 286.10525293 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Phenolic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- O-glycosyl compound
- Phenoxy compound
- Benzyl alcohol
- Phenol ether
- Monocyclic benzene moiety
- Monosaccharide
- Oxane
- Benzenoid
- Secondary alcohol
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Acetal
- Alcohol
- Hydrocarbon derivative
- Primary alcohol
- Aromatic alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 200 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | G933 |
|---|
| AKSci | J10775 |
|---|
| AKSci | HMDB0003546 |
|---|
| Cayman Chemical | 17357 |
|---|
| Glentham | GC4870 |
|---|
| MetaSci | HMDB0003546 |
|---|
| Toronto Research Chemicals | S087600 |
|---|