| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:10 UTC |
|---|
| Update date | 2017-01-19 02:36:39 UTC |
|---|
| FoodComEx ID | PC000872 |
|---|
| FoodDB Record | FDB023065 |
|---|
| Chemical Information |
|---|
| Name | Cortisone |
|---|
| Description | A naturally occurring glucocorticoid. It has been used in replacement therapy for adrenal insufficiency and as an anti-inflammatory agent. Cortisone itself is inactive. It is converted in the liver to the active metabolite hydrocortisone. (From Martindale, The Extra Pharmacopoeia, 30th ed, p726) -- Pubchem; Cortisone is a hormone. Chemically it is a corticosteroid with formula C21H28O5 and IUPAC name 17-hydroxy-11-dehydrocorticosterone. It is closely related to corticosterone. -- Wikipedia; One of cortisone's effects on the body, and a potentially harmful side effect when administered clinically, is the suppression of the immune system. This is an explanation for the apparent correlation between high stress and sickness. -- Wikipedia [HMDB] |
|---|
| CAS Number | 53-06-5 |
|---|
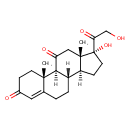
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 11-dehydro-17-Hydroxycorticosterone | ChEBI | | 17-Hydroxy-11-dehydrocorticosterone | ChEBI | | 17a,21-Dihydroxy-4-pregnene-3,11,20-trione | Generator | | 17alpha,21-Dihydroxy-4-pregnene-3,11,20-trione | ChEBI | | 17α,21-dihydroxy-4-pregnene-3,11,20-trione | Generator | | 4-Pregnene-17a,21-diol-3,11,20-trione | Generator | | 4-Pregnene-17alpha,21-diol-3,11,20-trione | ChEBI | | 4-Pregnene-17α,21-diol-3,11,20-trione | Generator | | Andreson | hmdb | | Anusol HC | hmdb | | Balneol-Hc | hmdb | | Beta-Hc | hmdb | | Colocort | hmdb | | Compound E | hmdb | | Corlin | hmdb | | Cortadren | hmdb | | Cortandren | hmdb | | Cortef | hmdb | | Cortef Acetate | hmdb | | Cortisal | hmdb | | Cortisate | hmdb | | Cortison | hmdb | | Cortisone Acetate | hmdb | | Cortistal | hmdb | | Cortivite | hmdb | | Cortogen | hmdb | | Cortone | hmdb | | Cortril | hmdb | | delta(4)-Pregnene-17a,21-diol-3,11,20-trione | Generator | | Delta(4)-Pregnene-17alpha,21-diol-3,11,20-trione | ChEBI | | Dermacort | hmdb | | Dricort | hmdb | | Flexicort | hmdb | | Florinef | hmdb | | Fludrocortisone Acetate | hmdb | | Glycort | hmdb | | Hemsol-Hc | hmdb | | Hi-Cor | hmdb | | Incortin | hmdb | | Kendall's compound | hmdb | | Kendall's Compound E | hmdb | | Kortison | ChEBI | | Locoid | hmdb | | Locoid Lipocream | hmdb | | Micort-Hc | hmdb | | Nogenic HC | hmdb | | Orabase HCA | hmdb | | Pandel | hmdb | | Pregn-4-en-17a,21-diol-3,11,20-trione | Generator | | Pregn-4-en-17alpha,21-diol-3,11,20-trione | ChEBI | | Pregn-4-en-17α,21-diol-3,11,20-trione | Generator | | Prestwick_132 | hmdb | | Reichstein Fa | hmdb | | Reichstein's Substance FA | hmdb | | Scheroson | hmdb | | Solu-Cortef | hmdb | | Stie-Cort | hmdb | | Texacort | hmdb | | Westcort | hmdb | | Wintersteiner's compound F | ChEBI | | δ(4)-pregnene-17a,21-diol-3,11,20-trione | Generator | | δ(4)-pregnene-17α,21-diol-3,11,20-trione | Generator |
|
|---|
| Chemical Formula | C21H28O5 |
|---|
| IUPAC name | (1S,2R,10S,11S,14R,15S)-14-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-ene-5,17-dione |
|---|
| InChI Identifier | InChI=1S/C21H28O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h9,14-15,18,22,26H,3-8,10-11H2,1-2H3/t14-,15-,18+,19-,20-,21-/m0/s1 |
|---|
| InChI Key | MFYSYFVPBJMHGN-ZPOLXVRWSA-N |
|---|
| Isomeric SMILES | [H][C@@]12CC[C@](O)(C(=O)CO)[C@@]1(C)CC(=O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| Average Molecular Weight | 360.444 |
|---|
| Monoisotopic Molecular Weight | 360.193674006 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- Pregnane-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- 11-oxosteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Cyclic alcohol
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Primary alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 50 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C592 |
|---|
| AKSci | HMDB0002802 |
|---|
| Glentham | GP7495 |
|---|
| MetaSci | HMDB0002802 |
|---|
| Toronto Research Chemicals | C696500 |
|---|