| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:08 UTC |
|---|
| Update date | 2017-01-19 02:36:39 UTC |
|---|
| FoodComEx ID | PC000865 |
|---|
| FoodDB Record | FDB015890 |
|---|
| Chemical Information |
|---|
| Name | Canthaxanthin |
|---|
| Description | Food colouring. Constituent of the edible mushroom (Cantharellus cinnabarinus), sea trout, salmon and brine shrimp. It is used in broiler chicken feed to enhance the yellow colour of chicken skin
Canthaxanthin (pronounced /?kæn???zæn??n/ ( listen)) is a carotenoid pigment widely distributed in nature. Carotenoids belong to a larger class of phytochemicals known as terpenoids. The chemical formula of canthaxanthin is C40H52O2. It has E number E161g.; Canthaxanthin is a food additive used for farmed salmon raised in environments where astaxanthin sources are not available. Canthaxanthin gives salmon a pink color similar to pink/red species of wild salmon, while at the same time acting as an antibiotic. It has E number E161g. -- Wikipedia; Canthaxanthin is not found in wild Atlantic Salmon, but is a minor carotenoid in Pacific Salmon. Canthaxanthin is used in farm-raised trout. Canthaxanthin is used in combination with astaxanthin for some salmon feeds.; The analysis of canthaxanthin content in salmon is a scientifically-accepted method to determine the origin of salmons. -- Wikipedia; Xanthophyll; A trans-carotenoid pigment widely distributed in nature. The compound is used as an oral suntanning agent and as a food and drug coloring agent. Oral ingestion of the compound causes canthaxanthin retinopathy. -- Pubchem |
|---|
| CAS Number | 514-78-3 |
|---|
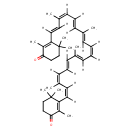
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 4,4'-Diketo-b-carotene | biospider | | 4,4'-Diketo-beta-carotene | biospider | | 4,4'-dioxo-b-Carotene | Generator | | 4,4'-Dioxo-beta-carotene | biospider | | 4,4'-dioxo-β-carotene | Generator | | all-trans-b-Carotene-4,4'-dione | Generator | | all-trans-beta-Carotene-4,4'-dione | ChEBI | | all-trans-β-carotene-4,4'-dione | Generator | | all-trans,beta-Carotene-4,4'-dione | biospider | | Aphanicin | db_source | | b,b-Carotene-4,4'-dione | db_source | | beta-Carotene-4,4'-dione | biospider | | beta-Carotene-4,4'-dione, all-trans- | biospider | | beta,beta-Carotene-4,4'-dione | biospider | | beta,beta-Carotene-4,4'-dione (9CI) | biospider | | C.I. Food Orange 8 | biospider | | Cantaxanthin | biospider | | Cantaxanthine | biospider | | Canthaxanthin | db_source | | CANTHAXANTHIN (SEE RETINOID PROJECT 1) | biospider | | Canthaxanthin (trans) | biospider | | Canthaxanthine | biospider | | Carophyll red | db_source | | Carotene-4,4'-dione, beta- | biospider | | Chlorellaxanthin | db_source | | e 161g | ChEBI | | E161g | db_source | | Euglenanone | db_source | | Food orange 8 | biospider | | L-Orange 7 | biospider | | Orobronze | biospider | | Roxanthin Red 10 | biospider |
|
|---|
| Chemical Formula | C40H52O2 |
|---|
| IUPAC name | 2,4,4-trimethyl-3-[(1Z,3Z,5E,7E,9Z,11E,13E,15Z,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethyl-3-oxocyclohex-1-en-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]cyclohex-2-en-1-one |
|---|
| InChI Identifier | InChI=1S/C40H52O2/c1-29(17-13-19-31(3)21-23-35-33(5)37(41)25-27-39(35,7)8)15-11-12-16-30(2)18-14-20-32(4)22-24-36-34(6)38(42)26-28-40(36,9)10/h11-24H,25-28H2,1-10H3/b12-11-,17-13+,18-14+,23-21-,24-22+,29-15+,30-16+,31-19-,32-20- |
|---|
| InChI Key | FDSDTBUPSURDBL-MFMWEGPCSA-N |
|---|
| Isomeric SMILES | C/C(/C=C/C=C(/C)\C=C\C1=C(C)C(=O)CCC1(C)C)=C\C=C/C=C(\C)/C=C/C=C(/C)\C=C/C1=C(C)C(=O)CCC1(C)C |
|---|
| Average Molecular Weight | 564.8397 |
|---|
| Monoisotopic Molecular Weight | 564.396730908 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Cyclohexenone
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 218° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | V1721 |
|---|
| Toronto Research Chemicals | C175735 |
|---|