| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:08 UTC |
|---|
| Update date | 2017-01-19 02:36:39 UTC |
|---|
| FoodComEx ID | PC000864 |
|---|
| FoodDB Record | FDB023587 |
|---|
| Chemical Information |
|---|
| Name | Gabapentin |
|---|
| Description | Gabapentin was originally developed as a chemical analogue of gamma-aminobutyric acid (GABA) to reduce the spinal reflex for the treatment of spasticity and was found to have anticonvulsant activity in various seizure models. In addition, it also displays antinociceptive activity in various animal pain models. Clinically, gabapentin is indicated as an add-on medication for the treatment of partial seizures, and neuropathic pain. It was also claimed to be beneficial in several other clinical disorders such as anxiety, bipolar disorder, and hot flashes. The possible mechanisms or targets involved in the multiple therapeutic actions of gabapentin have been actively studied. Since gabapentin was developed, several hypotheses had been proposed for its action mechanisms. They include selectively activating the heterodimeric GABA(B) receptors consisting of GABA(B1a) and GABA(B2) subunits, selectively enhancing the NMDA current at GABAergic interneurons, or blocking AMPA-receptor-mediated transmission in the spinal cord, binding to the L-alpha-amino acid transporter, activating ATP-sensitive K(+) channels, activating hyperpolarization-activated cation channels, and modulating Ca(2+) current by selectively binding to the specific binding site of [(3)H]gabapentin, the alpha(2)delta subunit of voltage-dependent Ca(2+) channels. Different mechanisms might be involved in different therapeutic actions of gabapentin. In this review, we summarized the recent progress in the findings proposed for the antinociceptive action mechanisms of gabapentin and suggest that the alpha(2)delta subunit of spinal N-type Ca(2+) channels is very likely the analgesic action target of gabapentin. (PMID: 16474201) [HMDB] |
|---|
| CAS Number | 60142-96-3 |
|---|
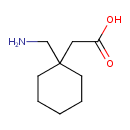
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1-(Aminomethyl)cyclohexaneacetate | Generator | | 1-(Aminomethyl)cyclohexaneacetic acid | hmdb | | Aclonium | hmdb | | Gabapentin | hmdb | | Gabapentina | ChEBI | | Gabapentine | hmdb | | Gabapentino | hmdb | | Gabapentinum | hmdb | | Gabapetin | hmdb | | Neurontin | hmdb | | Novo-Gabapentine | hmdb |
|
|---|
| Chemical Formula | C9H17NO2 |
|---|
| IUPAC name | 2-[1-(aminomethyl)cyclohexyl]acetic acid |
|---|
| InChI Identifier | InChI=1S/C9H17NO2/c10-7-9(6-8(11)12)4-2-1-3-5-9/h1-7,10H2,(H,11,12) |
|---|
| InChI Key | UGJMXCAKCUNAIE-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NCC1(CC(O)=O)CCCCC1 |
|---|
| Average Molecular Weight | 171.2368 |
|---|
| Monoisotopic Molecular Weight | 171.125928793 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as gamma amino acids and derivatives. These are amino acids having a (-NH2) group attached to the gamma carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Gamma amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gamma amino acid or derivatives
- Amino fatty acid
- Fatty acyl
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | F886 |
|---|
| AKSci | J10627 |
|---|
| AKSci | J91475 |
|---|
| Cayman Chemical | 10008346 |
|---|
| Glentham | GP0653 |
|---|
| Toronto Research Chemicals | G117250 |
|---|
| Toronto Research Chemicals | KIT1235 |
|---|