| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:05 UTC |
|---|
| Update date | 2017-01-19 02:36:39 UTC |
|---|
| FoodComEx ID | PC000851 |
|---|
| FoodDB Record | FDB022599 |
|---|
| Chemical Information |
|---|
| Name | Heparin |
|---|
| Description | A highly acidic mucopolysaccharide formed of equal parts of sulfated D-glucosamine and D-glucuronic acid with sulfaminic bridges. The molecular weight ranges from six to twenty thousand. Heparin occurs in and is obtained from liver, lung, mast cells, etc., of vertebrates. Its function is unknown, but it is used to prevent blood clotting in vivo and vitro, in the form of many different salts. [HMDB] |
|---|
| CAS Number | 9005-49-6 |
|---|
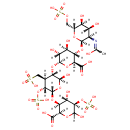
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| alpha-Heparin | hmdb | | Arteven | hmdb | | Bemiparin | hmdb | | Certoparin | hmdb | | Clexane | hmdb | | Clivarine | hmdb | | Dalteparin | hmdb | | Enoxaparin | hmdb | | Eparina | hmdb | | Fraxiparin | hmdb | | Heparin | hmdb | | Heparin Sodium | hmdb | | Heparin sulfate | hmdb | | Heparin sulphate | HMDB | | Heparinate | hmdb | | Heparinic acid | hmdb | | Heparinsodiumsalt | hmdb | | Thromboliquine | hmdb |
|
|---|
| Chemical Formula | C26H41NO34S4 |
|---|
| IUPAC name | (2S,3S,4R,5R,6R)-6-{[(2S,3S,4S,5R,6S)-6-{[(2R,3S,4S,5R)-2-carboxy-4,6-dihydroxy-5-(sulfooxy)oxan-3-yl]oxy}-2-hydroxy-4-(sulfomethyl)-5-(sulfooxy)oxan-3-yl]oxy}-3-{[(2R,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-[(sulfooxy)methyl]oxan-2-yl]oxy}-4,5-dihydroxyoxane-2-carboxylic acid |
|---|
| InChI Identifier | InChI=1S/C26H41NO34S4/c1-4(28)27-7-9(30)8(29)6(2-52-63(43,44)45)53-24(7)56-15-10(31)11(32)25(58-19(15)21(36)37)55-13-5(3-62(40,41)42)14(60-64(46,47)48)26(59-22(13)38)57-16-12(33)17(61-65(49,50)51)23(39)54-18(16)20(34)35/h5-19,22-26,29-33,38-39H,2-3H2,1H3,(H,27,28)(H,34,35)(H,36,37)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)/t5-,6+,7+,8+,9+,10+,11+,12-,13-,14+,15-,16-,17+,18+,19-,22-,23?,24+,25+,26-/m0/s1 |
|---|
| InChI Key | ZFGMDIBRIDKWMY-PASTXAENSA-N |
|---|
| Isomeric SMILES | CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@@H]2[C@@H](O)O[C@H](O[C@H]3[C@H](O)[C@@H](OS(O)(=O)=O)C(O)O[C@H]3C(O)=O)[C@H](OS(O)(=O)=O)[C@H]2CS(O)(=O)=O)O[C@@H]1C(O)=O |
|---|
| Average Molecular Weight | 1039.85 |
|---|
| Monoisotopic Molecular Weight | 1039.039280225 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as n-acyl-alpha-hexosamines. These are carbohydrate derivatives containing a hexose moiety in which the oxygen atom is replaced by an n-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acyl-alpha-hexosamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl glycoside
- Fatty acyl glycoside of mono- or disaccharide
- N-acyl-alpha-hexosamine
- Disaccharide sulfate
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- O-glycosyl compound
- Disaccharide
- Glycosyl compound
- Fatty acyl
- Dicarboxylic acid or derivatives
- Sulfuric acid ester
- Alkyl sulfate
- Sulfate-ester
- Sulfuric acid monoester
- Oxane
- Pyran
- Acetamide
- Alkanesulfonic acid
- Organic sulfuric acid or derivatives
- Sulfonyl
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Carboxamide group
- Hemiacetal
- Secondary alcohol
- Secondary carboxylic acid amide
- Oxacycle
- Carboxylic acid derivative
- Carboxylic acid
- Organoheterocyclic compound
- Acetal
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Alcohol
- Organosulfur compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 10 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | K633 |
|---|