| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:02 UTC |
|---|
| Update date | 2017-01-19 02:36:38 UTC |
|---|
| FoodComEx ID | PC000843 |
|---|
| FoodDB Record | FDB000505 |
|---|
| Chemical Information |
|---|
| Name | L-Canavanine |
|---|
| Description | Stored in large quantities in the seeds of leguminous plants in three subfamilies. Isol. originally from Jackbean (Canavalia ensiformis)
L-(+)-(S)-Canavanine is a non-proteinogenic amino acid of certain leguminous plants. It is structurally related to the proteinogenic amino acid, L-arginine. Canavanine is accumulated primarily in the seeds where it serves both as a defensive compound against herbivores and a vital source of nitrogen for the growing embryo. Organisms that consume it can mistakenly incorporate it into their own proteins in the place of arginine, thereby producing structurally aberrant proteins that may not function properly. Some specialized herbivores tolerate L-canavanine either because they metabolize it efficiently or avoid its incorporation into their own nascent proteins.; L-Canavanine, a non-protein amino acid of certain leguminous plants, is related structurally to the protein amino acid, L-arginine. Canavanine is accumulated primarily in the seeds where it serves both as a defensive compound against herbivores and a vital source of nitrogen for the growing embryo. Organisms that consume it can mistakenly incorporate it into their own proteins, in the place of arginine thereby producing structurally aberrant proteins that may not function properly or not at all. Some specialized herbivores tolerate L-canavanine either because they metabolize it efficiently or avoid its incorporation into their own nascent proteins. -- Wikipedia (unverified). L-Canavanine is found in many foods, some of which are mamey sapote, purslane, hard wheat, and cabbage. |
|---|
| CAS Number | 543-38-4 |
|---|
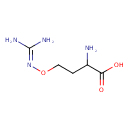
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S)-2-Ammonio-4-(carbamimidamidooxy)butanoate | ChEBI | | (2S)-2-Ammonio-4-(carbamimidamidooxy)butanoic acid | Generator | | (l)-canavanine | biospider | | 2-Amino-4-(guanidinooxy)butyric acid | biospider | | 543-38-4 (FREE BASE) | biospider | | Butyric acid, 2-amino-4-(guanidinooxy)-, L | biospider | | Butyric acid, 2-amino-4-(guanidinooxy)-, L- | biospider | | Canavanin | biospider | | Canavanine | biospider | | Canavanine; L-form | db_source | | GGB | biospider | | L-2-AMINO-4-(GUANIDINOOXY)BUTYRIC ACID | biospider | | L-canavanine | biospider | | L-canavanine sulfate | biospider | | L-homoserine, o-((aminoiminomethyl)amino)- | biospider | | L-Homoserine, O-((aminoiminomethyl)amino)- (9CI) | biospider | | L(+)-canavanine | biospider | | NSC8921 (SULFATE) | biospider | | O-((aminoiminomethyl)amino)-l-homoserine | biospider | | O-((aminoiminomethyl)amino)homoserine | biospider |
|
|---|
| Chemical Formula | C5H12N4O3 |
|---|
| IUPAC name | 2-amino-4-{[(diaminomethylidene)amino]oxy}butanoic acid |
|---|
| InChI Identifier | InChI=1S/C5H12N4O3/c6-3(4(10)11)1-2-12-9-5(7)8/h3H,1-2,6H2,(H,10,11)(H4,7,8,9) |
|---|
| InChI Key | FSBIGDSBMBYOPN-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC(CCON=C(N)N)C(O)=O |
|---|
| Average Molecular Weight | 176.1738 |
|---|
| Monoisotopic Molecular Weight | 176.09094027 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Fatty acid
- Guanidine
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Amine
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -3.324 | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 184° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 40 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | X6876 |
|---|