| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:01 UTC |
|---|
| Update date | 2017-01-19 02:36:38 UTC |
|---|
| FoodComEx ID | PC000838 |
|---|
| FoodDB Record | FDB022406 |
|---|
| Chemical Information |
|---|
| Name | N-Acetylaspartylglutamic acid |
|---|
| Description | N-Acetylaspartylglutamate (NAAG) is a neuropeptide found in millimolar concentrations in brain that is localized to subpopulations of glutamatergic, cholinergic, GABAergic, and noradrenergic neuronal systems. NAAG is released upon depolarization by a Ca(2+)-dependent process and is an agonist at mGluR3 receptors and an antagonist at NMDA receptors. NAAG is catabolized to N-acetylaspartate and glutamate primarily by glutamate carboxypeptidase II, which is expressed on the extracellular surface of astrocytes. The levels of NAAG and the activity of carboxypeptidase II are altered in a regionally specific fashion in several neuropsychiatric disorders. (PMID 9361299)
N-Acetylaspartylglutamic acid (NAAG) is a purported precursor of N-Acetylaspartic acid (NAA) and is present at about one-tenth of the concentration of NAA in the brain. NAAG has been reported to activate N-methyl- D-aspartic acid (NMDA) receptors in neurons. Previous immunohistochemical studies in the vertebrate central nervous system (CNS) have suggested that NAAG is exclusively localized to neurons. Recent evidence, however, indicates that NAAG might also be localized to nonneuronal cells within the CNS. Only traces of NAA and NAAG are detectable in other tissues. Some compounds can change levels of NAA and NAAG in the brain. For example, methylphenidante increases the levels of NAA and NAAG in the cerebral cortex; amphetamine also increases NAA concentration in a mature brain by 26%, raising the possibility that other neurochemical systems might be involved in the clinical effects of stimulants. (PMID: 10603234) [HMDB] |
|---|
| CAS Number | 3106-85-2 |
|---|
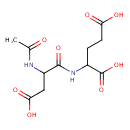
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| a-Spaglumic acid | hmdb | | Acetyl-a-L-aspartylglutamic acid | hmdb | | Acetyl-alpha-L-aspartylglutamic acid | hmdb | | alpha-Spaglumic acid | hmdb | | Isospaglumic acid | hmdb | | N-(N-Acetylaspartyl)glutamic acid | hmdb | | N-Acetyl-a-aspartylglutamic acid | hmdb | | N-Acetyl-a-L-aspartyl-L-glutamic acid | hmdb | | N-Acetyl-alpha-aspartylglutamic acid | hmdb | | N-Acetyl-alpha-L-aspartyl-L-glutamic acid | hmdb | | N-Acetyl-L-aspartyl-L-glutamic acid | hmdb | | NAAG | hmdb |
|
|---|
| Chemical Formula | C11H16N2O8 |
|---|
| IUPAC name | 2-(3-carboxy-2-acetamidopropanamido)pentanedioic acid |
|---|
| InChI Identifier | InChI=1S/C11H16N2O8/c1-5(14)12-7(4-9(17)18)10(19)13-6(11(20)21)2-3-8(15)16/h6-7H,2-4H2,1H3,(H,12,14)(H,13,19)(H,15,16)(H,17,18)(H,20,21) |
|---|
| InChI Key | OPVPGKGADVGKTG-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CC(=O)NC(CC(O)=O)C(=O)NC(CCC(O)=O)C(O)=O |
|---|
| Average Molecular Weight | 304.2533 |
|---|
| Monoisotopic Molecular Weight | 304.090665498 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Glutamic acid or derivatives
- Aspartic acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Tricarboxylic acid or derivatives
- Fatty amide
- Fatty acyl
- N-acyl-amine
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 8896AJ |
|---|
| Toronto Research Chemicals | S679100 |
|---|