| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:57 UTC |
|---|
| Update date | 2017-01-19 02:36:37 UTC |
|---|
| FoodComEx ID | PC000827 |
|---|
| FoodDB Record | FDB022126 |
|---|
| Chemical Information |
|---|
| Name | Deuteroporphyrin IX |
|---|
| Description | Deuteroporphyrin IX is a non-natural dicarboxylic porphyrin. Deuteroporphyrins are porphyrins with four methyl and two propionic acid side chains attached to the pyrrole rings. Deuteroporphyrin IX is described as a fecal porphyrin in patients with endemic chronic arsenic poisoning. (Environmental Sciences (Tokyo, Japan) (2001), 8(6), 561-570.)
Excess accumulation of the biosynthetic intermediate protoporphyrin can lead to extensive tissue damage upon exposure to light since protoporpyhyrin is a potent photosensitizing agent, giving rise to membrane-destroying oxygen radicals or singlet oxygen. For instance, in the human porphyria disease porphyria variegata, a genetic deficiency in a heme biosynthetic enzyme, protoporphyrinogen oxidase, leads to protoporphyrin accumulation and lightdependent skin photosensitivity. Horseradish peroxidase (HRP) in the presence of glutathione (GSH) could oxidize the non-natural porphyrin deuteroporphyrin IX, which is closely related to protoporphyrin IX. The product is a unique green chlorin. One of the pyrrole rings had been modified by addition of an hydroxy and an oxo group, thus giving the characteristic reduced pyrrole ring of the chlorine. Of most importance for human medicine, peroxidative enzymes present in mammalian cells can also carry out these GSH-dependent oxidative conversions of protoporphyrin and deuteroporphyrin. (PMID: 10334939) [HMDB] |
|---|
| CAS Number | 448-65-7 |
|---|
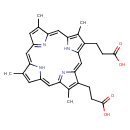
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Deuteroporphyrin | hmdb | | Deuteroporphyrin-IX | hmdb |
|
|---|
| Chemical Formula | C30H30N4O4 |
|---|
| IUPAC name | 3-[20-(2-carboxyethyl)-5,9,14,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoic acid |
|---|
| InChI Identifier | InChI=1S/C30H30N4O4/c1-15-9-20-12-25-17(3)21(5-7-29(35)36)27(33-25)14-28-22(6-8-30(37)38)18(4)26(34-28)13-24-16(2)10-19(32-24)11-23(15)31-20/h9-14,31,34H,5-8H2,1-4H3,(H,35,36)(H,37,38)/b19-11-,20-12-,23-11-,24-13-,25-12-,26-13-,27-14-,28-14- |
|---|
| InChI Key | KWKUIRGQJJFUCG-LMKUSPAJSA-N |
|---|
| Isomeric SMILES | CC1=C/C2=C/C3=N/C(=C\C4=C(CCC(O)=O)C(C)=C(N4)/C=C4\N=C(C=C4C)\C=C\1/N\2)/C(CCC(O)=O)=C3C |
|---|
| Average Molecular Weight | 510.5836 |
|---|
| Monoisotopic Molecular Weight | 510.226705468 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 20 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |