| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:55 UTC |
|---|
| Update date | 2017-01-19 02:36:37 UTC |
|---|
| FoodComEx ID | PC000820 |
|---|
| FoodDB Record | FDB000546 |
|---|
| Chemical Information |
|---|
| Name | L-Pipecolic acid |
|---|
| Description | Present in beans and other legumes, and in lesser quantities in other plants including barley, hops, malt and mushrooms. L-Pipecolic acid is found in many foods, some of which are macadamia nut (m. tetraphylla), linden, tinda, and cumin. |
|---|
| CAS Number | 3105-95-1 |
|---|
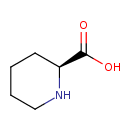
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-Pipecolate | biospider | | (-)-Pipecolic acid | biospider | | (2S)-Piperidine-2-carboxylic acid | biospider | | (S)-(-)-2-Piperidinecarboxylate | biospider | | (S)-(-)-2-Piperidinecarboxylic acid | biospider | | (s)-(-)-pipecolate | biospider | | (s)-(-)-pipecolic acid | biospider | | (S)-2-Piperidinecarboxylate | biospider | | (S)-2-Piperidinecarboxylic acid | biospider | | (s)-pipecolate | biospider | | (s)-pipecolic acid | biospider | | (s)-pipecolinate | biospider | | (s)-pipecolinic acid | biospider | | (S)-Piperidine-2-carboxylate | biospider | | (S)-Piperidine-2-carboxylic acid | biospider | | 2-Piperidinecarboxylic acid, 9CI; L-form | db_source | | L-(-)-pipecolate | biospider | | L-(-)-pipecolic acid | biospider | | L-homoproline | biospider | | L-pipecolate | biospider | | L-pipecolic acid | biospider | | L-pipecolinate | biospider | | L-pipecolinic acid | biospider | | L-Piperidine-2-carboxylate | biospider | | L-Piperidine-2-carboxylic acid | biospider | | Pipecolic acid, (s)-(-)- | biospider | | Pipecolic acid, l- | biospider | | Pipecolic acid, l-(-)- | biospider |
|
|---|
| Chemical Formula | C6H11NO2 |
|---|
| IUPAC name | (2S)-piperidine-2-carboxylic acid |
|---|
| InChI Identifier | InChI=1S/C6H11NO2/c8-6(9)5-3-1-2-4-7-5/h5,7H,1-4H2,(H,8,9)/t5-/m0/s1 |
|---|
| InChI Key | HXEACLLIILLPRG-YFKPBYRVSA-N |
|---|
| Isomeric SMILES | OC(=O)[C@@H]1CCCCN1 |
|---|
| Average Molecular Weight | 129.157 |
|---|
| Monoisotopic Molecular Weight | 129.078978601 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Piperidinecarboxylic acid
- Piperidine
- Amino acid
- Carboxylic acid
- Secondary aliphatic amine
- Monocarboxylic acid or derivatives
- Secondary amine
- Organoheterocyclic compound
- Azacycle
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Carbonyl group
- Organopnictogen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 259-260° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | E692 |
|---|
| AKSci | J92544 |
|---|
| Toronto Research Chemicals | P479750 |
|---|