| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:52 UTC |
|---|
| Update date | 2017-01-19 02:36:37 UTC |
|---|
| FoodComEx ID | PC000812 |
|---|
| FoodDB Record | FDB013380 |
|---|
| Chemical Information |
|---|
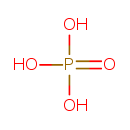
| Name | Phosphoric acid |
|---|
| Description | Phosphoric acid or free phosphate, also known as H3PO4, belongs to the class of inorganic compounds known as non-metal phosphates. These are inorganic non-metallic compounds containing a phosphate as its largest oxoanion. Phosphate is a moderately acidic compound (based on its pKa). When phosphorous pentoxide is dissolved in water it produces phosphoric acid. Phosphoric acid is a colorless, odorless phosphorus-containing inorganic acid with a bland taste. Phosphate, in the form of phosphoric acid, phosphate ions (H2PO4 or HPO4 – at neutral pH) or various salts of phosphate, exists in all living species, ranging from bacteria to plants to humans. Free orthophosphate anions can be released by the hydrolysis of the phosphoanhydride bonds in ATP or ADP. These phosphorylation and dephosphorylation reactions are the immediate storage and source of energy for many metabolic processes. ATP and ADP are often referred to as high-energy phosphates. An important occurrence of phosphates in biological systems is as the structural material of bone and teeth. These structures are made of crystalline calcium phosphate in the form of hydroxyapatite. Vitamin D, which helps stimulate bone growth and stability, stimulates phosphate absorption, an effect reported to precede its action on calcium ion transport. Phosphate supplementation of the diet of rodents has been shown to lead to reduction in the incidence of dental caries and different phosphates have different powers in reducing the cariogenic potential of the carbohydrates in a diet. Food-grade phosphoric acid (additive E338) is used to acidify foods and beverages such as various colas and jams, providing a tangy or sour taste. Soft drinks containing phosphoric acid, including Coca-Cola, are sometimes called phosphate sodas or phosphates. Phosphoric acid also has the potential to contribute to the formation of kidney stones, especially in those who have had kidney stones previously (PMID:25364887). Phosphate, in the form of phosphoric acid, is one of the simple acids found in cannabis plant (PMID: 6991645). |
|---|
| CAS Number | 7664-38-2 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| [PO(OH)3] | ChEBI | | Acide orique | ChEBI | | Acidum oricum | ChEBI | | Diate tetrasodium | HMDB | | E338 | db_source | | FEMA 2900 | db_source | | H3PO4 | ChEBI | | Marphos | HMDB | | NFB | HMDB | | o-Phosphoric acid | biospider | | Orate | Generator | | oric acid | ChEBI | | Orsaeure | ChEBI | | Orsaeureloesungen | ChEBI | | ortho- Oric acid | HMDB | | orthoate | Generator | | orthoic acid | Generator | | orthooric acid | ChEBI | | Orthophosphoric acid | db_source | | Sodium pyroate | HMDB | | Sodium pyroate decahydrate | HMDB | | Sodium pyroate decahydrate biochemica | HMDB | | Sonac | HMDB | | Tetra-sodium pyroate | HMDB | | Tetrasodium pyroate 10-hydrate | HMDB | | Tetrasodium pyroate decahydrate | HMDB | | Trihydroxo oxophosphorus(V) | db_source | | White oric acid | HMDB |
|
|---|
| Chemical Formula | H3O4P |
|---|
| IUPAC name | phosphoric acid |
|---|
| InChI Identifier | InChI=1S/H3O4P/c1-5(2,3)4/h(H3,1,2,3,4) |
|---|
| InChI Key | NBIIXXVUZAFLBC-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OP(O)(O)=O |
|---|
| Average Molecular Weight | 97.9952 |
|---|
| Monoisotopic Molecular Weight | 97.976895096 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of inorganic compounds known as non-metal phosphates. These are inorganic non-metallic compounds containing a phosphate as its largest oxoanion. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Non-metal oxoanionic compounds |
|---|
| Sub Class | Non-metal phosphates |
|---|
| Direct Parent | Non-metal phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Non-metal phosphate
- Inorganic oxide
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -1.436 | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 42.3° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Glentham | GK4212 |
|---|
| Toronto Research Chemicals | P359200 |
|---|