| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:47 UTC |
|---|
| Update date | 2017-01-19 02:36:37 UTC |
|---|
| FoodComEx ID | PC000805 |
|---|
| FoodDB Record | FDB022780 |
|---|
| Chemical Information |
|---|
| Name | 3,7-Dimethyluric acid |
|---|
| Description | 3,7-Dimethyluric acid, also known as 3,7-dimethylate or 3,7-DMU, belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. An oxopurine that is 7,9-dihydro-1H-purine-2,6,8(3H)-trione substituted by methyl groups at N-3 and N-7. 3,7-Dimethyluric acid is an extremely weak basic (essentially neutral) compound (based on its pKa). 3,7-Dimethyluric acid exists in all living organisms, ranging from bacteria to humans. 3,7-dimethyluric acid can be biosynthesized from theobromine through the action of the enzyme xanthine dehydrogenase/oxidase. In humans, 3,7-dimethyluric acid is involved in caffeine metabolism. |
|---|
| CAS Number | 13087-49-5 |
|---|
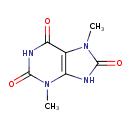
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 3,7-Dimethyl-2,6,8-trihydroxypurine | hmdb | | 3,7-dimethyl-7,9-dihydro-1H-purine-2,6,8(3H)-trione | hmdb | | 7-Dimethylate | Generator | | 7-Dimethylic acid | Generator |
|
|---|
| Chemical Formula | C7H8N4O3 |
|---|
| IUPAC name | 3,7-dimethyl-2,3,6,7,8,9-hexahydro-1H-purine-2,6,8-trione |
|---|
| InChI Identifier | InChI=1S/C7H8N4O3/c1-10-3-4(8-6(10)13)11(2)7(14)9-5(3)12/h1-2H3,(H,8,13)(H,9,12,14) |
|---|
| InChI Key | HMLZLHKHNBLLJD-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CN1C(=O)NC2=C1C(=O)NC(=O)N2C |
|---|
| Average Molecular Weight | 196.1634 |
|---|
| Monoisotopic Molecular Weight | 196.059640142 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- 6-oxopurine
- Purinone
- Alkaloid or derivatives
- Pyrimidone
- N-substituted imidazole
- Pyrimidine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Urea
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 100 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |