| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:40 UTC |
|---|
| Update date | 2017-01-19 02:36:36 UTC |
|---|
| FoodComEx ID | PC000790 |
|---|
| FoodDB Record | FDB022258 |
|---|
| Chemical Information |
|---|
| Name | Dimethylprotoporphyrin IX dimethyl ester |
|---|
| Description | The hepatic pigment accumulated as a consequence of the self-catalyzed destruction of cytochrome P-450 by norethisterone (PubMed ID 284396 ) [HMDB] |
|---|
| CAS Number | 5522-66-7 |
|---|
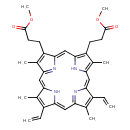
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Dimethyl 3,8,13,17-tetramethyl-7,12-divinyl-21H,23H-porphine-2,18-dipropionate | hmdb | | Dimethyl 7,12-diethenyl-3,8,13,17-tetramethyl-21H,23H-porphine-2,18-dipropanoate | hmdb | | Dimethyl 7,12-diethenyl-3,8,13,17-tetramethyl-21H,23H-porphine-2,18-dipropanoic acid | hmdb | | Dimethyl Protoporphyrin IX | hmdb | | Protoporphyrin dimethyl ester | hmdb | | Protoporphyrin IX di-Me ester | hmdb | | Protoporphyrin IX dimethyl ester | hmdb |
|
|---|
| Chemical Formula | C36H38N4O4 |
|---|
| IUPAC name | methyl 3-[9,14-diethenyl-20-(3-methoxy-3-oxopropyl)-5,10,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoate |
|---|
| InChI Identifier | InChI=1S/C36H38N4O4/c1-9-23-19(3)27-15-28-21(5)25(11-13-35(41)43-7)33(39-28)18-34-26(12-14-36(42)44-8)22(6)30(40-34)17-32-24(10-2)20(4)29(38-32)16-31(23)37-27/h9-10,15-18,37,40H,1-2,11-14H2,3-8H3/b27-15-,28-15-,29-16-,30-17-,31-16-,32-17-,33-18-,34-18- |
|---|
| InChI Key | WASRLAPXOHTNAX-MFBGAUBSSA-N |
|---|
| Isomeric SMILES | COC(=O)CCC1=C2NC(\C=C3/N=C(/C=C4\N\C(=C/C5=N/C(=C\2)/C(CCC(=O)OC)=C5C)C(C)=C4C=C)C(C)=C3C=C)=C1C |
|---|
| Average Molecular Weight | 590.7113 |
|---|
| Monoisotopic Molecular Weight | 590.289305724 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 100 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | V0222 |
|---|
| Toronto Research Chemicals | P838900 |
|---|