| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:40 UTC |
|---|
| Update date | 2017-01-19 02:36:36 UTC |
|---|
| FoodComEx ID | PC000789 |
|---|
| FoodDB Record | FDB022372 |
|---|
| Chemical Information |
|---|
| Name | N-Formyl-L-methionine |
|---|
| Description | Effective in the initiation of protein synthesis. The initiating methionine residue enters the ribosome as N-formylmethionyl tRNA. This process occurs in Escherichia coli and other bacteria as well as in the mitochondria of eucaryotic cells. [HMDB] |
|---|
| CAS Number | 4289-98-9 |
|---|
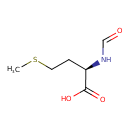
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| fMet | hmdb | | For-Met-OH | hmdb | | Formyl-L-methionine | hmdb | | formylmethionine | hmdb | | N-Formyl-L-methionine | hmdb | | N-formyl-methionine | hmdb |
|
|---|
| Chemical Formula | C6H11NO3S |
|---|
| IUPAC name | (2R)-2-formamido-4-(methylsulfanyl)butanoic acid |
|---|
| InChI Identifier | InChI=1S/C6H11NO3S/c1-11-3-2-5(6(9)10)7-4-8/h4-5H,2-3H2,1H3,(H,7,8)(H,9,10)/t5-/m1/s1 |
|---|
| InChI Key | PYUSHNKNPOHWEZ-RXMQYKEDSA-N |
|---|
| Isomeric SMILES | CSCC[C@@H](NC=O)C(O)=O |
|---|
| Average Molecular Weight | 177.221 |
|---|
| Monoisotopic Molecular Weight | 177.045963913 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as methionine and derivatives. Methionine and derivatives are compounds containing methionine or a derivative thereof resulting from reaction of methionine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Methionine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methionine or derivatives
- N-formyl-alpha-amino acid
- N-formyl-alpha amino acid or derivatives
- Thia fatty acid
- Fatty acyl
- Fatty acid
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Thioether
- Sulfenyl compound
- Dialkylthioether
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 200 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | V5922 |
|---|
| MetaSci | HMDB0001015 |
|---|
| Sigma-Aldrich | HMDB0001015 |
|---|
| Toronto Research Chemicals | F700693 |
|---|