| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:40 UTC |
|---|
| Update date | 2017-01-19 02:36:36 UTC |
|---|
| FoodComEx ID | PC000786 |
|---|
| FoodDB Record | FDB022726 |
|---|
| Chemical Information |
|---|
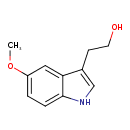
| Name | 5-Methoxytryptophol |
|---|
| Description | 5-Methoxytryptophol is synthesized by the pineal gland. Daily rhythms in pineal methoxyindole metabolism have been described in rodents and humans (5-Methoxytryptophol levels are coincident with serotonin levels in rodents pineal) and 5-Methoxytryptophol at its highest during the daylight hours and fall markedly soon after the onset of darkness, coincident with increases in the levels of pineal melatonin and the activities of pineal serotonin-N-acetyltransferase (EC 2.3.1.87, SNAT) and hydroxyindole-O-methyltransferase (EC 2.1.1.4, HIOMT). The fact that the levels of 5-methoxytryptophol and melatonin vary in parallel suggests that the major factor generating the methoxyindole rhythms is not SNAT activity, but perhaps a change in the availability (for metabolism) of "stored" serotonin. When the onset of darkness is delayed by 12 hours, human 5-methoxytryptophol (and melatonin) rhythms usually require 3 or 4 days to adjust to the new lighting regimen. Environmental factors, other than light, that activate the sympathetic nervous system or cause epinephrine to be secreted from the adrenal medulla (e.g., the stress of immobilization; insulin-induced hypoglycemia) can override the inhibitory effects of light and accelerate melatonin synthesis. Rhythms in 5-methoxytryptophol (and melatonin) synthesis apparently persist among animals placed in environments of continuous darkness; the source of the cyclic signal (mediated by the pineal sympathetic nerves) has not yet been identified. Preliminary evidence suggests that levels of a peptide hormone, arginine vasotocin, in rat pineal and sera also exhibit daily rhythms and are increased by norepinephrine. The circadian rhythm of melatonin secretion is generated in the suprachiasmatic nucleus. Sleep disruption, nightly restlessness, sundowning, and other circadian disturbances are frequently seen in Alzheimer's disease patients. Changes in the suprachiasmatic nucleus and pineal gland are thought to be the biological basis for these behavioral disturbances. (PMID 288858, 2245336) [HMDB] |
|---|
| CAS Number | 712-09-4 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 5-Methoxy-1H-indole-3-ethanol | hmdb | | 5-Methoxyindole-3-ethanol | hmdb | | 5-Methoxytryptophol | hmdb | | Methoxytryptophol | hmdb |
|
|---|
| Chemical Formula | C11H13NO2 |
|---|
| IUPAC name | 2-(5-methoxy-1H-indol-3-yl)ethan-1-ol |
|---|
| InChI Identifier | InChI=1S/C11H13NO2/c1-14-9-2-3-11-10(6-9)8(4-5-13)7-12-11/h2-3,6-7,12-13H,4-5H2,1H3 |
|---|
| InChI Key | QLWKTGDEPLRFAT-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | COC1=CC=C2NC=C(CCO)C2=C1 |
|---|
| Average Molecular Weight | 191.2264 |
|---|
| Monoisotopic Molecular Weight | 191.094628665 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 3-alkylindoles. 3-Alkylindoles are compounds containing an indole moiety that carries an alkyl chain at the 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indoles |
|---|
| Direct Parent | 3-alkylindoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-alkylindole
- Anisole
- Alkyl aryl ether
- Substituted pyrrole
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Ether
- Azacycle
- Hydrocarbon derivative
- Organopnictogen compound
- Alcohol
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 20 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | Q693 |
|---|
| Cayman Chemical | 21061 |
|---|
| Toronto Research Chemicals | M271710 |
|---|