| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:39 UTC |
|---|
| Update date | 2017-01-19 02:36:36 UTC |
|---|
| FoodComEx ID | PC000782 |
|---|
| FoodDB Record | FDB023116 |
|---|
| Chemical Information |
|---|
| Name | Guanidinosuccinic acid |
|---|
| Description | Guanidinosuccinic acid (GSA) is one of the earliest uremic toxins isolated and its toxicity identified. Its metabolic origins show that it arose from the oxidation of argininosuccinic acid (ASA) by free radicals. The stimulus for this oxidation, occurring optimally in the presence of the failed kidney, is the rising level of urea which, through enzyme inhibition, results in a decline in hepatic levels of the semi-essential amino acid, arginine. It is further noted that concentrations of GSA in both serum and urine decline sharply in animals and humans exposed to the essential amino acid, methionine. Uremic patients suffer from a defective ability to generate methyl groups due to anorexia, dietary restrictions and renal protein leakage. This leads to the accumulation of homocysteine, a substance known to produce vascular damage. Even in healthy subjects intake of choline together with methionine is insufficient to satisfy total metabolic requirements for methyl groups. In end-stage renal disease, therefore, protein restriction contributes to the build-up of toxins in uremia. Replacement using specific amino acid mixtures should be directed toward identified deficiencies and adequacy monitored by following serum levels of the related toxins, in this case GSA and homocysteine. (PMID 12701806) [HMDB] |
|---|
| CAS Number | 6133-30-8 |
|---|
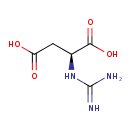
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S)-2-Carbamimidamidobutanedioate | Generator | | (2S)-2-Carbamimidamidobutanedioic acid | ChEBI | | Guanidinosuccinate | hmdb | | L-N-Amidinoaspartate | Generator | | L-N-amidinoaspartic acid | hmdb | | N-(Aminoiminomethyl)-L-aspartate | Generator | | N-(aminoiminomethyl)-L-aspartic acid | hmdb | | N-Amidino-L-aspartate | hmdb | | N-amidino-L-aspartic acid | hmdb | | N-carbamimidoyl-L-aspartic acid | hmdb |
|
|---|
| Chemical Formula | C5H9N3O4 |
|---|
| IUPAC name | (2S)-2-carbamimidamidobutanedioic acid |
|---|
| InChI Identifier | InChI=1S/C5H9N3O4/c6-5(7)8-2(4(11)12)1-3(9)10/h2H,1H2,(H,9,10)(H,11,12)(H4,6,7,8)/t2-/m0/s1 |
|---|
| InChI Key | VVHOUVWJCQOYGG-REOHCLBHSA-N |
|---|
| Isomeric SMILES | NC(=N)N[C@@H](CC(O)=O)C(O)=O |
|---|
| Average Molecular Weight | 175.1427 |
|---|
| Monoisotopic Molecular Weight | 175.059305791 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as aspartic acid and derivatives. Aspartic acid and derivatives are compounds containing an aspartic acid or a derivative thereof resulting from reaction of aspartic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Aspartic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aspartic acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acid
- Guanidine
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboxylic acid
- Organopnictogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 15 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |