| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:39 UTC |
|---|
| Update date | 2017-01-19 02:36:36 UTC |
|---|
| FoodComEx ID | PC000781 |
|---|
| FoodDB Record | FDB023107 |
|---|
| Chemical Information |
|---|
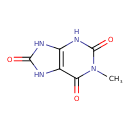
| Name | 1-Methyluric acid |
|---|
| Description | 1-Methyluric acid, also known as 1-methylate, belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. An oxopurine that is 7,9-dihydro-1H-purine-2,6,8(3H)-trione substituted by a methyl group at N-1. 1-Methyluric acid is an extremely weak basic (essentially neutral) compound (based on its pKa). 1-Methyluric acid exists in all living organisms, ranging from bacteria to humans. 1-methyluric acid can be biosynthesized from 1-methylxanthine through the action of the enzyme xanthine dehydrogenase/oxidase. In humans, 1-methyluric acid is involved in caffeine metabolism. |
|---|
| CAS Number | 708-79-2 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1-Methylate | Generator | | 1-Methylic acid | Generator | | 1-Methylurate | hmdb |
|
|---|
| Chemical Formula | C6H6N4O3 |
|---|
| IUPAC name | 1-methyl-2,3,6,7,8,9-hexahydro-1H-purine-2,6,8-trione |
|---|
| InChI Identifier | InChI=1S/C6H6N4O3/c1-10-4(11)2-3(9-6(10)13)8-5(12)7-2/h1H3,(H,9,13)(H2,7,8,12) |
|---|
| InChI Key | QFDRTQONISXGJA-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CN1C(=O)NC2=C(NC(=O)N2)C1=O |
|---|
| Average Molecular Weight | 182.1368 |
|---|
| Monoisotopic Molecular Weight | 182.043990078 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- 6-oxopurine
- Purinone
- Alkaloid or derivatives
- Pyrimidone
- Pyrimidine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Urea
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 10 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| MetaSci | HMDB0003099 |
|---|
| Sigma-Aldrich | HMDB0003099 |
|---|
| Toronto Research Chemicals | M338180 |
|---|