| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:39 UTC |
|---|
| Update date | 2017-01-19 02:36:36 UTC |
|---|
| FoodComEx ID | PC000780 |
|---|
| FoodDB Record | FDB003166 |
|---|
| Chemical Information |
|---|
| Name | Hydroxycobalamin |
|---|
| Description | Vitamin (haematopoietic)
A B 12 vitamin. It has been used therapeutically in the treatment and prevention of vitamin B 12 deficiency. -- Pubchem; Hydroxocobalamin (OHCbl) is a natural analog of vitamin B-12, a basic member of the cobalamin family of compounds. It has an intense red color. Vitamin B12 is a term that refers to a group of compounds called cobalamins that are available in the human body in a variety of mostly interconvertible forms. Together with folic acid, cobalamins are essential cofactors required for DNA synthesis in cells where chromosomal replication and division are occurring?most notably the bone marrow and myeloid cells. As a cofactor, cobalamins are essential for two cellular reactions: (1) the mitochondrial methylmalonylcoenzyme A mutase conversion of methylmalonic acid (MMA) to succinate, which links lipid and carbohydrate metabolism, and (2) activation of methionine synthase, which is the rate limiting step in the synthesis of methionine from homocysteine and tetrahydrofolate (Katzung, 1989).; Hydroxocobalamin is a synthetic, injectable form of Vitamin B12. Hydroxocobalamin is actually a precursor of two cofactors or vitamins (Vitamin B12 and Methylcobalamin) which are involved in various biological systems in man. Vitamin B12 is required for the conversion of methylmalonate to succinate. Deficiency of this enzyme could therefore interfere with the production of lipoprotein in myelin sheath tissue and so give rise to neurological lesions. The second cofactor, Methylcobalamin, is necessary for the conversion of homocysteine to methionine which is essential for the metabolism of folic acid. Deficiency of tetrahydrafolate leads to reduced synthesis of thymidylate resulting in reduced synthesis of DNA which is essential for cell maturation. Vitamin B12 is also concerned in the maintenance of sulphydryl groups in reduced form, deficiency leading to decreased amounts of reduced SH content of erythrocytes and liver cells. Overall, vitamin B12 acts as a coenzyme for various metabolic functions, including fat and carbohydrate metabolism and protein synthesis. It is necessary for growth, cell replication, hematopoiesis, and nucleoprotein as well as myelin synthesis. This is largely due to its effects on metabolism of methionine folic acid, and malonic acid. |
|---|
| CAS Number | 13422-51-0 |
|---|
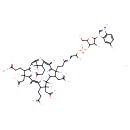
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| a-(5,6-Dimethylbenzimidazolyl)hydroxocobamide | biospider | | Alpha cobione | biospider | | alpha-(5,6-Dimethylbenzimidazolyl)hydroxocobamide | biospider | | AlphaRedisol | db_source | | Axlon | biospider | | Ciplamin h | biospider | | Cobalex | biospider | | Cobalin h | biospider | | Cobinamide dihydroxide dihydrogen phosphate (ester) mono (inner salt) 3'-ester with 5,6-dimethyl -1-a-D-ribofuranosyl-1H-benzimidazole, 9CI | db_source | | Cobinamide hydroxide ate 3'-ester with 5,6-dimethyl-1-a-D-ribofuranosylbenzimidazole inner salt | HMDB | | Cobinamide hydroxide ate 3'-ester with 5,6-dimethyl-1-alpha-delta-ribofuranosylbenzimidazole inner salt | HMDB | | Codroxomin | db_source | | Docclan | biospider | | Docelan | biospider | | Docevita | biospider | | Droxomin | biospider | | Ducobee HY | biospider | | Duradoce | biospider | | Duralta-12 | biospider | | Hydrocobalamin | biospider | | Hydrogrisevit | biospider | | Hydrovit | biospider | | Hydroxocobalamin | db_source | | Hydroxocobalamine | biospider | | Hydroxycobalamin | biospider | | Hydroxycobalamin, BAN, INN, USAN | db_source | | Lipflavonoid | db_source | | Neo-Betalin 12 | db_source | | Oh-CBL | biospider | | Sytobex H | db_source | | Vibeden | db_source | | vitamin B-12b | biospider |
|
|---|
| Chemical Formula | C62H89CoN13O15P |
|---|
| IUPAC name | cobalt(3+) ion 3-[13-(3-azanidyl-3-oxopropyl)-4-{2-[(2-{[hydroxy({[4-hydroxy-2-(hydroxymethyl)-5-(5-methyl-1-methylidene-3H-1lambda5,3-benzodiazol-3-yl)oxolan-3-yl]oxy})phosphoryl]oxy}propyl)-C-hydroxycarbonimidoyl]ethyl}-18-[2-(C-hydroxycarbonimidoyl)ethyl]-3,14,19-tris[(C-hydroxycarbonimidoyl)methyl]-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1^{2,5}.1^{7,10}.1^{12,15}]tricosa-5(23),6,10(22),11,15(21),16-hexaen-8-yl]propanecarboximidate hydrate |
|---|
| InChI Identifier | InChI=1S/C62H90N13O14P.Co.H2O/c1-30-12-16-39-40(22-30)75(29-74(39)11)57-52(84)53(41(28-76)87-57)89-90(85,86)88-31(2)27-69-49(83)20-21-59(7)37(23-46(66)80)56-62(10)61(9,26-48(68)82)36(15-19-45(65)79)51(73-62)33(4)55-60(8,25-47(67)81)34(13-17-43(63)77)38(70-55)24-42-58(5,6)35(14-18-44(64)78)50(71-42)32(3)54(59)72-56;;/h12,16,22,24,29,31,34-37,41,52-53,56-57,76,84H,11,13-15,17-21,23,25-28H2,1-10H3,(H15,63,64,65,66,67,68,69,70,71,72,73,77,78,79,80,81,82,83,85,86);;1H2/q;+3;/p-3 |
|---|
| InChI Key | OHTROZZULXOIDW-UHFFFAOYSA-K |
|---|
| Isomeric SMILES | O=C(CCC1C2=C(C3=[N]4C(=CC5=[N]6C(C(C5(C)C)CCC(=O)N)=C(C5=[N]7C8C(N2[Co+]467(C[N]2=CN(C4=CC(C)=CC=C24)C2OC(CO)C(OP(=O)(OC(C)CNC(=O)CCC5(C)C8CC(=O)N)[O-])C2O)O)(C1(CC(=O)N)C)C)C)C(CCC(N)=O)C3(CC(=O)N)C)C)N |
|---|
| Average Molecular Weight | 1346.3551 |
|---|
| Monoisotopic Molecular Weight | 1345.567070949 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cobalamin derivatives. These are organic compounds containing a corrin ring, a cobalt atom, an a nucleotide moiety. Cobalamin Derivatives are actually derived from vitamin B12. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Corrinoids |
|---|
| Direct Parent | Cobalamin derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cobalamin
- Metallotetrapyrrole skeleton
- Tertiary aromatic amine
- Pentose phosphate
- N-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Diaminotoluene
- Benzimidazole
- Dialkyl phosphate
- Benzenoid
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Monosaccharide
- Tetrahydrofuran
- Pyrroline
- Pyrrolidine
- Tertiary amine
- Secondary alcohol
- Ketimine
- Amino acid or derivatives
- Oxacycle
- Azacycle
- Organic transition metal salt
- Organic 1,3-dipolar compound
- Carbene-type 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Enamine
- Secondary aliphatic amine
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Carboxylic acid amidine
- Amidine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic cobalt salt
- Organic salt
- Organic zwitterion
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Imine
- Carbonyl group
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | 200 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | V1817 |
|---|
| Cayman Chemical | 24099 |
|---|
| Toronto Research Chemicals | H862820 |
|---|