| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:35 UTC |
|---|
| Update date | 2017-01-19 02:36:35 UTC |
|---|
| FoodComEx ID | PC000771 |
|---|
| FoodDB Record | FDB021982 |
|---|
| Chemical Information |
|---|
| Name | 17alpha-Hydroxypregnenolone |
|---|
| Description | 17a-Hydroxypregnenolone is a 21-carbon steroid that is converted from pregnenolone by cytochrome P450 17alpha hydroxylase/C17,20 lyase (CYP17, EC 1.14.99.9). 17a-Hydroxypregnenolone is an intermediate in the delta-5 pathway of biosynthesis of gonadal steroid hormones and the adrenal corticosteroids. The first, rate-limiting and hormonally regulated step in the biosynthesis of all steroid hormones is the conversion of cholesterol to pregnenolone. The conversion of cholesterol to pregnenolone is accomplished by the cleavage of the cholesterol side chain, catalyzed by a mitochondrial cytochrome P450 enzyme termed P450scc where scc designates Side Chain Cleavage. All steroid hormones are made from the pregnenolone produced by P450scc; thus, the presence or absence of each of the activities of CYP17 directs this pregnenolone towards its final metabolic pathway. While all cytochrome P450 enzymes can catalyze multiple reactions on a single active site, CYP17 is the only one described to date in which these multiple activities are differentially regulated by a physiologic process. 17a-Hydroxypregnenolone is converted to dehydroepiandrosterone by the 17,20 lyase activity of CYP17. The ratio of the 17,20 lyase to 17 alpha-hydroxylase activity of CYP17 determines the ratio of C21 to C19 steroids produced. This ratio is regulated post-translationally by at least three factors: the abundance of the electron-donating protein P450 oxidoreductase, the presence of cytochrome b5, and the serine phosphorylation of CYP17. (PMID: 12573809) [HMDB] |
|---|
| CAS Number | 387-79-1 |
|---|
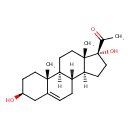
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (3b)-3,17-Dihydroxypregn-5-en-20-one | Generator | | (3beta)-3,17-Dihydroxypregn-5-en-20-one | ChEBI | | (3β)-3,17-dihydroxypregn-5-en-20-one | Generator | | 17-Hydroxy-D5-pregnenolone | hmdb | | 17-Hydroxypregnenolone | hmdb | | 17-OH-pregnenolone | hmdb | | 17a-Hydroxypregnenolone | Generator | | 17a-Hydroxypregnolone | hmdb | | 17alpha-Hydroxypregnanolone | hmdb | | 17alpha-Hydroxypregnenolone | hmdb | | 17α-hydroxypregnenolone | Generator | | 3b,17-Dihydroxy-5-pregnen-20-one | hmdb | | 3b,17-Dihydroxy-pregn-5-en-20-one | hmdb | | 3b,17a-Dihydroxypregn-5-en-20-one | hmdb | | 5-Pregnen-3b,17a-diol-20-one | hmdb | | 5-Pregnen-3beta,17alpha-diol-20-one | ChEBI | | 5-Pregnen-3β,17α-diol-20-one | Generator |
|
|---|
| Chemical Formula | C21H32O3 |
|---|
| IUPAC name | 1-[(1S,2R,5S,10R,11S,14R,15S)-5,14-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-14-yl]ethan-1-one |
|---|
| InChI Identifier | InChI=1S/C21H32O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h4,15-18,23-24H,5-12H2,1-3H3/t15-,16+,17-,18-,19-,20-,21-/m0/s1 |
|---|
| InChI Key | JERGUCIJOXJXHF-TVWVXWENSA-N |
|---|
| Isomeric SMILES | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |
|---|
| Average Molecular Weight | 332.477 |
|---|
| Monoisotopic Molecular Weight | 332.23514489 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- 17-hydroxysteroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Oxosteroid
- Hydroxysteroid
- Delta-5-steroid
- Alpha-hydroxy ketone
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 10 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | N721 |
|---|
| Toronto Research Chemicals | H952320 |
|---|