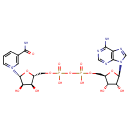
| [(3S,2R,4R,5R)-5-(6-Aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl {[(3S,2R,4R,5R)-5-(3-carbamoylpyridyl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxyoryl) hydrogen ate | HMDB |
| [(3S,2R,4R,5R)-5-(6-Aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl {[(3S,2R,4R,5R)-5-(3-carbamoylpyridyl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxyphosphoryl) hydrogen phosphate | hmdb |
| [adenylate-32-P]-NAD | hmdb |
| 3-Carbamoyl-1-beta-D-ribofuranosylpyridinium hydroxide 5'-ester with adenosine 5'-pyroate inner salt | HMDB |
| 3-Carbamoyl-1-beta-D-ribofuranosylpyridinium hydroxide 5'-ester with adenosine 5'-pyrophosphate inner salt | hmdb |
| 3-Carbamoyl-1-beta-delta-ribofuranosylpyridinium hydroxide 5'-ester with adenosine 5'-pyroate inner salt | HMDB |
| 3-Carbamoyl-1-beta-delta-ribofuranosylpyridinium hydroxide 5'-ester with adenosine 5'-pyrophosphate inner salt | hmdb |
| 3-Carbamoyl-1-D-ribofuranosylpyridinium hydroxide 5'-ester with adenosine 5'-pyroate | HMDB |
| 3-Carbamoyl-1-D-ribofuranosylpyridinium hydroxide 5'-ester with adenosine 5'-pyrophosphate | hmdb |
| 3-Carbamoyl-1-delta-ribofuranosylpyridinium hydroxide 5'-ester with adenosine 5'-pyroate | HMDB |
| 3-Carbamoyl-1-delta-ribofuranosylpyridinium hydroxide 5'-ester with adenosine 5'-pyrophosphate | hmdb |
| Adenine-nicotinamide dinucleotide | hmdb |
| beta-Diopyridine nucleotide | HMDB |
| beta-Diphosphopyridine nucleotide | hmdb |
| beta-NAD | hmdb |
| beta-Nicotinamide adenine dinucleotide | hmdb |
| beta-Nicotinamide adenine dinucleotide trihydrate | hmdb |
| CO-I | hmdb |
| Codehydrase I | hmdb |
| Codehydrogenase I | hmdb |
| Coenzyme I | hmdb |
| Cozymase | hmdb |
| Cozymase I | hmdb |
| Diopyridine nucleotide | ChEBI |
| Diopyridine nucleotide oxidized | HMDB |
| Diphosphopyridine nucleotide | hmdb |
| diphosphopyridine nucleotide oxidized | hmdb |
| DPN | ChEBI |
| Endopride | hmdb |
| NAD trihydrate | hmdb |
| NAD-oxidized | hmdb |
| NAD+ | ChEBI |
| Nadide | ChEBI |
| Nicotinamide adenine dinucleotide | hmdb |
| nicotinamide adenine dinucleotide oxidized | hmdb |
| Nicotinamide dinucleotide | hmdb |
| Nicotineamide adenine dinucleotide | hmdb |
| Oxidized diopyridine nucleotide | HMDB |
| Oxidized diphosphopyridine nucleotide | hmdb |
| Pyridine nucleotide diate | HMDB |
| Pyridine nucleotide diphosphate | hmdb |