| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:33 UTC |
|---|
| Update date | 2017-01-19 02:36:35 UTC |
|---|
| FoodComEx ID | PC000761 |
|---|
| FoodDB Record | FDB023199 |
|---|
| Chemical Information |
|---|
| Name | Scopolamine |
|---|
| Description | Scopolamine, also known as hyoscine, is a tropane alkaloid drug obtained from plants of the family Solanaceae (nightshades), such as henbane or jimson weed (Datura species). It is part of the secondary metabolites of plants.
Scopolamine is used criminally as a date rape drug and as an aid to robbery, the most common act being the clandestine drugging of a victim's drink. It is preferred because it induces retrograde amnesia, or an inability to recall events prior to its administration. Victims of this crime are often admitted to a hospital in police custody, under the assumption that the patient is experiencing a psychotic episode. A telltale sign is a fever accompanied by a lack of sweat.
An alkaloid from Solanaceae, especially Datura metel L. and Scopola carniolica. Scopolamine and its quaternary derivatives act as antimuscarinics like atropine, but may have more central nervous system effects. Among the many uses are as an anesthetic premedication, in urinary incontinence, in motion sickness, as an antispasmodic, and as a mydriatic and cycloplegic. [HMDB] |
|---|
| CAS Number | 51-34-3 |
|---|
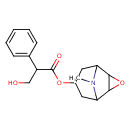
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-Hyoscine | hmdb | | (-)-Hyoscine hydrobromide | hmdb | | (-)-Scopolamine | ChEBI | | (-)-Scopolamine bromide | hmdb | | (-)-Scopolamine hydrobromide | hmdb | | (+)-Hyoscine | hmdb | | (+)-Scopolamine | hmdb | | (1S,3S,5R,6R,7S)-6,7-Epoxytropan-3-yl (2S)-3-hydroxy-2-phenylpropanoate | ChEBI | | (1S,3S,5R,6R,7S)-6,7-Epoxytropan-3-yl (2S)-3-hydroxy-2-phenylpropanoic acid | Generator | | 6-b,7-b-Epoxy-3-a-tropanyl S-(-)-tropate | Generator | | 6-b,7-b-Epoxy-3-a-tropanyl S-(-)-tropic acid | Generator | | 6-beta,7-beta-Epoxy-3-alpha-tropanyl S-(-)-tropate | ChEBI | | 6-beta,7-beta-Epoxy-3-alpha-tropanyl S-(-)-tropic acid | Generator | | 6-β,7-β-epoxy-3-α-tropanyl S-(-)-tropate | Generator | | 6-β,7-β-epoxy-3-α-tropanyl S-(-)-tropic acid | Generator | | 6,7-Epoxytropine Tropate | hmdb | | 6,7-Epoxytropine tropic acid | Generator | | a-(Hydroxymethyl)benzeneacetate 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | a-(Hydroxymethyl)benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | alpha-(Hydroxymethyl)benzeneacetate 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | alpha-(Hydroxymethyl)benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | ChEBI | | Atrochin | hmdb | | Atroquin | hmdb | | Beldavrin | hmdb | | Buscopan | hmdb | | Epoxytropine tropate | hmdb | | Euscopol | hmdb | | Hydroscine hydrobromide | hmdb | | Hyosceine | hmdb | | Hyoscine | ChEBI | | Hyoscine bromide | hmdb | | Hyoscine hydrobromide | hmdb | | Hyoscyine hydrobromide | hmdb | | Hyosol | hmdb | | Hysco | hmdb | | Isopto Hyoscine | hmdb | | Isoscopil | hmdb | | Kwells | hmdb | | L-Hyoscine hydrobromide | hmdb | | l-Scopolamine-hydrobromide | hmdb | | Methscopolamine Bromide | hmdb | | Oscine | hmdb | | Pamine | hmdb | | S-(-)-Tropate | hmdb | | Scop | hmdb | | Scopamin | hmdb | | Scopine (-)-tropate | ChEBI | | Scopine (-)-tropic acid | Generator | | Scopine tropate | hmdb | | Scopolamine bromide | hmdb | | Scopolamine hydrobromide | hmdb | | Scopolaminium bromide | hmdb | | Scopolammonium bromide | hmdb | | SEE | hmdb | | Tranaxine | hmdb | | Transderm-scop | ChEBI | | α-(hydroxymethyl)benzeneacetate 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | α-(hydroxymethyl)benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator |
|
|---|
| Chemical Formula | C17H21NO4 |
|---|
| IUPAC name | 9-methyl-3-oxa-9-azatricyclo[3.3.1.0^{2,4}]nonan-7-yl 3-hydroxy-2-phenylpropanoate |
|---|
| InChI Identifier | InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3 |
|---|
| InChI Key | STECJAGHUSJQJN-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CN1C2CC(CC1C1OC21)OC(=O)C(CO)C1=CC=CC=C1 |
|---|
| Average Molecular Weight | 303.3529 |
|---|
| Monoisotopic Molecular Weight | 303.147058165 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-hydroxy acid
- Monocyclic benzene moiety
- Morpholine
- Oxazinane
- Piperidine
- N-alkylpyrrolidine
- Benzenoid
- Pyrrolidine
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary amine
- Tertiary aliphatic amine
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Dialkyl ether
- Oxirane
- Ether
- Monocarboxylic acid or derivatives
- Primary alcohol
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 100 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | S200005 |
|---|