| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:32 UTC |
|---|
| Update date | 2017-01-19 02:36:35 UTC |
|---|
| FoodComEx ID | PC000755 |
|---|
| FoodDB Record | FDB022614 |
|---|
| Chemical Information |
|---|
| Name | Coenzyme A |
|---|
| Description | Coenzyme A (CoA, CoASH, or HSCoA) is a coenzyme, notable for its role in the synthesis and oxidization of fatty acids, and the oxidation of pyruvate in the citric acid cycle. It is adapted from beta-mercaptoethylamine, panthothenate and adenosine triphosphate. Acetyl-CoA is an important molecule itself. It is the precursor to HMG CoA, which is a vital component in cholesterol and ketone synthesis. Furthermore, it contributes an acetyl group to choline to produce acetylcholine, in a reaction catalysed by choline acetyltransferase. Its main task is conveying the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. -- Wikipedia [HMDB]. Coenzyme A is found in many foods, some of which are grape, cowpea, pili nut, and summer savory. |
|---|
| CAS Number | 85-61-0 |
|---|
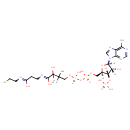
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| [(2R,3S,4R,5R)-5-(6-amino-9H-Purin-9-yl)-4-hydroxy-3-(onooxy)tetrahydrofuran-2-yl]methyl 3-hydroxy-4-({3-oxo-3-[(2-sulfanylethyl)amino]propyl}amino)-2,2-dimethyl-4-oxobutyl dihydrogen diate | HMDB | | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]methyl 3-hydroxy-4-({3-oxo-3-[(2-sulfanylethyl)amino]propyl}amino)-2,2-dimethyl-4-oxobutyl dihydrogen diphosphate | hmdb | | Acetoacetyl coenzyme A sodium salt | hmdb | | CoA | hmdb | | CoA hydrate | hmdb | | CoA-SH | hmdb | | CoASH | hmdb | | Coenzyme A | hmdb | | Coenzyme A hydrate | hmdb | | Coenzyme A-SH | hmdb | | Coenzyme ASH | hmdb | | coenzymes A | hmdb | | Depot-Zeel | hmdb | | Propionyl CoA | hmdb | | Propionyl Coenzyme A | hmdb | | S-propanoate | hmdb | | S-propanoate CoA | hmdb | | S-propanoate Coenzyme A | hmdb | | S-propanoic acid | hmdb | | S-propionate CoA | hmdb | | S-propionate Coenzyme A | hmdb | | Zeel | hmdb |
|
|---|
| Chemical Formula | C21H36N7O16P3S |
|---|
| IUPAC name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-2,2-dimethyl-3-({2-[(2-sulfanylethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| InChI Identifier | InChI=1S/C21H36N7O16P3S/c1-21(2,16(31)19(32)24-4-3-12(29)23-5-6-48)8-41-47(38,39)44-46(36,37)40-7-11-15(43-45(33,34)35)14(30)20(42-11)28-10-27-13-17(22)25-9-26-18(13)28/h9-11,14-16,20,30-31,48H,3-8H2,1-2H3,(H,23,29)(H,24,32)(H,36,37)(H,38,39)(H2,22,25,26)(H2,33,34,35)/t11-,14-,15-,16?,20-/m1/s1 |
|---|
| InChI Key | RGJOEKWQDUBAIZ-DRCCLKDXSA-N |
|---|
| Isomeric SMILES | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)C(O)C(=O)NCCC(=O)NCCS |
|---|
| Average Molecular Weight | 767.534 |
|---|
| Monoisotopic Molecular Weight | 767.115208365 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as coenzyme a and derivatives. These are derivative of vitamin B5 containing a 4'-phosphopantetheine moiety attached to a diphospho-adenosine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleotides |
|---|
| Sub Class | Purine ribonucleotides |
|---|
| Direct Parent | Coenzyme A and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monoalkyl phosphate
- Fatty amide
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Fatty acyl
- Imidolactam
- Alkyl phosphate
- Phosphoric acid ester
- Pyrimidine
- Tetrahydrofuran
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Alkylthiol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxide
- Organic nitrogen compound
- Hydrocarbon derivative
- Organosulfur compound
- Alcohol
- Amine
- Organopnictogen compound
- Primary amine
- Organic oxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | Not Available |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Cayman Chemical | 16147 |
|---|
| Glentham | GK0114 |
|---|