| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:18 UTC |
|---|
| Update date | 2017-01-19 02:36:34 UTC |
|---|
| FoodComEx ID | PC000734 |
|---|
| FoodDB Record | FDB023154 |
|---|
| Chemical Information |
|---|
| Name | Chitin |
|---|
| Description | Chitin is an unusual substance as it is a naturally occurring polymer. Its breakdown is conducted by bacteria which have receptors to simple sugars from the decomposition of chitin. If chitin is detected they then produce enzymes to digest the chitin by reducing it to simple sugars and ammonia.
Chitin (IPA: [Kaitin]) is one of the main components in the cell walls of fungi, the exoskeletons of insects and other arthropods, and in some other animals. It is a polysaccharide; it is constructed from units of acetylglucosamine (more completely, N-acetyl-D-glucos-2-amine). These are linked together in beta-1,4 fashion (in a similar manner to the glucose units which form cellulose). In effect chitin may be described as cellulose with one hydroxyl group on each monomer replaced by an acetylamine group. This allows for increased hydrogen bonding between adjacent polymers, giving the polymer increased strength.
A linear polysaccharide of beta-1->4 linked units of acetylglucosamine. It is the second most abundant biopolymer on earth, found especially in insects and fungi. When deacetylated it is called chitosan. [HMDB] |
|---|
| CAS Number | 1398-61-4 |
|---|
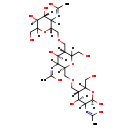
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| [1,4-(N-Acetyl-beta-D-glucosaminyl)]n | hmdb | | [1,4-(N-Acetyl-beta-D-glucosaminyl)]n+1 | hmdb | | [1,4-(N-Acetyl-beta-delta-glucosaminyl)]n | hmdb | | [1,4-(N-Acetyl-beta-delta-glucosaminyl)]n+1 | hmdb | | beta-1,4-Poly-N-acetyl-D-glucosamine | hmdb | | beta-1,4-Poly-N-acetyl-delta-glucosamine | hmdb | | Poly 2-Acetamido-2-deoxy-D-glucose | hmdb | | Poly 2-Acetamido-2-deoxy-delta-glucose | hmdb |
|
|---|
| Chemical Formula | C28H49N3O16 |
|---|
| IUPAC name | N-[(2R,4S,5S)-5-({[(2R,4S,5S)-3-acetamido-5-({[(2R,4R,5S)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]methoxy}methyl)-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]methoxy}methyl)-2,4-dihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide |
|---|
| InChI Identifier | InChI=1S/C28H49N3O16/c1-11(35)29-21-19(9-44-8-15-17(5-33)47-28(42)23(25(15)39)31-13(3)37)45-16(4-32)14(24(21)38)7-43-10-20-22(30-12(2)36)27(41)26(40)18(6-34)46-20/h14-28,32-34,38-42H,4-10H2,1-3H3,(H,29,35)(H,30,36)(H,31,37)/t14-,15-,16?,17?,18?,19+,20+,21?,22?,23?,24+,25+,26-,27-,28-/m1/s1 |
|---|
| InChI Key | DJHJJVWPFGHIPH-OODMECLYSA-N |
|---|
| Isomeric SMILES | CC(=O)NC1[C@H](O)OC(CO)[C@@H](COC[C@@H]2OC(CO)[C@@H](COC[C@@H]3OC(CO)[C@@H](O)[C@H](O)C3NC(C)=O)[C@H](O)C2NC(C)=O)[C@@H]1O |
|---|
| Average Molecular Weight | 683.6992 |
|---|
| Monoisotopic Molecular Weight | 683.311282535 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | C-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - C-glycosyl compound
- Monosaccharide
- Oxane
- Acetamide
- Carboxamide group
- Hemiacetal
- Secondary alcohol
- Secondary carboxylic acid amide
- Ether
- Dialkyl ether
- Carboxylic acid derivative
- Organoheterocyclic compound
- Oxacycle
- Organic nitrogen compound
- Primary alcohol
- Organic oxide
- Organonitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 400 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | G953 |
|---|
| Glentham | GC0425 |
|---|
| MetaSci | HMDB0003362 |
|---|
| Sigma-Aldrich | HMDB0003362 |
|---|
| Toronto Research Chemicals | C300800 |
|---|