| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:17 UTC |
|---|
| Update date | 2017-01-19 02:36:34 UTC |
|---|
| FoodComEx ID | PC000732 |
|---|
| FoodDB Record | FDB001161 |
|---|
| Chemical Information |
|---|
| Name | D-Galacturonic acid |
|---|
| Description | obtained from the hydrolysis prods. of polymers of pectic substances
D-Galacturonic acid is a sugar acid, an oxidized form of D-galactose. It is the main component of pectin, in which it exists as the polymer polygalacturonic acid. It has an aldehyde group at C1 and a carboxylic acid group at C6. Other oxidized forms of D-galactose are D-galactonic acid (carboxylic group at C1) and meso-galactaric acid (mucic acid) (carboxylic groups at C1 and C6). It is also a uronic acid or hexuronic acid. Naturally occurring uronic acids are D-glucuronic acid, D-galacturonic acid, L-iduronic acid and D-mannuronic acid.; D-Galacturonic acid is a sugar acid, the oxidized form of D-galactose. It is the main component of pectin, in which it exists as the polymer polygalacturonic acid. -- Wikipedia. D-Galacturonic acid is found in many foods, some of which are guava, yellow wax bean, common pea, and green bean. |
|---|
| CAS Number | 14982-50-4 |
|---|
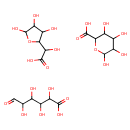
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S,3R,4S,5R)-2,3,4,5-Tetrahydroxy-6-oxohexanoate | Generator | | (2S,3R,4S,5R)-2,3,4,5-tetrahydroxy-6-oxohexanoic acid | biospider | | 3,5-Dideoxy-5-((hydroxyacetyl)amino)-D-glycero-D-galacto-2-nonulosonate | HMDB | | 3,5-Dideoxy-5-((hydroxyacetyl)amino)-D-glycero-D-galacto-2-nonulosonic acid | HMDB | | Aldehydo-D-galacturonate | Generator | | Aldehydo-d-galacturonic acid | biospider | | D-galactopyranuronic acid | biospider | | D-Galacturonate | Generator | | D-Galacturonic acid | biospider | | D-galacturonic acid, homopolymer | biospider | | DL-galacturonic acid | biospider | | Galactopyranuronic acid, d- | biospider | | Galacturonic acid | biospider | | Galacturonic acid, d- | biospider | | N-Glycolyl-5-neuraminate | HMDB | | N-Glycolyl-5-neuraminic acid | HMDB | | N-Glycolyl-b-neuraminate | Generator | | N-Glycolyl-b-neuraminic acid | Generator | | N-Glycolyl-beta-neuraminate | Generator | | N-Glycolyl-beta-neuraminic acid | ChEBI | | N-Glycolyl-β-neuraminate | Generator | | N-Glycolyl-β-neuraminic acid | Generator | | N-Glycolylneuraminic acid | ChEBI | | Neu5gc | ChEBI |
|
|---|
| Chemical Formula | C18H30O21 |
|---|
| IUPAC name | 2,3,4,5-tetrahydroxy-6-oxohexanoic acid; 2-hydroxy-2-(3,4,5-trihydroxyoxolan-2-yl)acetic acid; 3,4,5,6-tetrahydroxyoxane-2-carboxylic acid |
|---|
| InChI Identifier | InChI=1S/3C6H10O7/c7-1-2(8)6(12)13-4(1)3(9)5(10)11;7-1-2(8)4(5(10)11)13-6(12)3(1)9;7-1-2(8)3(9)4(10)5(11)6(12)13/h2*1-4,6-9,12H,(H,10,11);1-5,8-11H,(H,12,13) |
|---|
| InChI Key | TYNHVGLYUDYTJD-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(C=O)C(O)C(O)C(O)C(O)=O.OC(C1OC(O)C(O)C1O)C(O)=O.OC1OC(C(O)C(O)C1O)C(O)=O |
|---|
| Average Molecular Weight | 582.4182 |
|---|
| Monoisotopic Molecular Weight | 582.127958022 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as glucuronic acid derivatives. Glucuronic acid derivatives are compounds containing a glucuronic acid moiety (or a derivative), which consists of a glucose moiety with the C6 carbon oxidized to a carboxylic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Glucuronic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glucuronic acid or derivatives
- Medium-chain hydroxy acid
- Medium-chain fatty acid
- Hydroxy fatty acid
- Beta-hydroxy acid
- Alpha-hydroxy acid
- Beta-hydroxy aldehyde
- Hydroxy acid
- Monosaccharide
- Fatty acid
- Fatty acyl
- Pyran
- Oxane
- Tetrahydrofuran
- Alpha-hydroxyaldehyde
- Hemiacetal
- Secondary alcohol
- Oxacycle
- Carboxylic acid derivative
- Carboxylic acid
- Organoheterocyclic compound
- Polyol
- Monocarboxylic acid or derivatives
- Carbonyl group
- Aldehyde
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 156-159 dec. (sinters at 110°) | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |