| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:13 UTC |
|---|
| Update date | 2017-01-19 02:36:34 UTC |
|---|
| FoodComEx ID | PC000723 |
|---|
| FoodDB Record | FDB023068 |
|---|
| Chemical Information |
|---|
| Name | Alloxan |
|---|
| Description | Alloxan or mesoxalylurea is an organic compound based on a pyrimidine heterocyclic skeleton. This compound has a high affinity for water and therefore exists as the monohydrate. The compound was discovered by Justus von Liebig and Friedrich Wohler following the discovery of urea in 1828 and is one of the oldest named organic compounds that exist. The name is derived from allantoin, a product of uric acid excreted by the fetus into the allantois and oxaluric acid derived from oxalic acid and urea, found in urine. The original recipe for Alloxan was by oxidation of uric acid by nitric acid. Alloxan is a strong oxidizing agent and it forms a hemiacetal with its reduced reaction product dialuric acid (in which a carbonyl group is reduced to a hydroxyl group) which is called alloxantin. -- Wikipedia; Alloxane is a raw material for the production of the purple dye Murexide. Carl Wilhelm Scheele discovered the dye in 1776. Murexide is the product of the complex in-situ multistep reaction of alloxantin and gaseous ammonia. Murexide results from the condensation of the unisolated intermdiate uramil with alloxan, liberated during the course of the reaction. Scheele sourced uric acid from human calculi (such as kidney stones) and called the compound lithic acid. William Prout investigated the compound in 1818 and he used boa constrictor excrement with up to 90% ammonium acid urate. Liebig and Wohler in the nineteenth century coined the name murexide for the dye after the Trunculus Murex which is the source of the Tyrian purple of antiquity. Primo Levi in his world famous novel The Periodic Table in chapter Nitrogen considers pythons as a source for alloxane on behalf of a lipstick producer but he is turned down by the director of the Turin zoo because the zoo already has lucrative contracts with cosmetics companies (his attempts with chicken dung end in misery). -- Wikipedia [HMDB] |
|---|
| CAS Number | 50-71-5 |
|---|
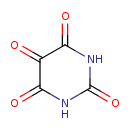
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2,4,5,6-Pyrimidinetetrone | ChEBI | | 2,4,5,6-Pyrimidintetron | hmdb | | 2,4,5,6-Pyrimidintetrone | hmdb | | 2,4,5,6-Tetraoxohexahydropyrimidine | hmdb | | 2,4,5,6(1H,3H)-Pyrimidinetetrone | hmdb | | 5-oxo-barbiturate | hmdb | | 5-oxo-barbituric acid | hmdb | | 5-Oxobarbitate | Generator | | 5-Oxobarbitic acid | Generator | | 5-Oxobarbituric acid | ChEBI | | 5,6-Dioxouracil | ChEBI | | alloxan | hmdb | | Alloxan 7169 | hmdb | | Alloxan tetrahydrat | hmdb | | Alloxane | hmdb | | mesoxalyl-Urea | hmdb | | Mesoxalylcarbamide | hmdb | | Mesoxalylurea | hmdb | | NSC 7169 | ChEBI | | Pyrimidinetetrone | hmdb |
|
|---|
| Chemical Formula | C4H2N2O4 |
|---|
| IUPAC name | 1,3-diazinane-2,4,5,6-tetrone |
|---|
| InChI Identifier | InChI=1S/C4H2N2O4/c7-1-2(8)5-4(10)6-3(1)9/h(H2,5,6,8,9,10) |
|---|
| InChI Key | HIMXGTXNXJYFGB-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | O=C1NC(=O)C(=O)C(=O)N1 |
|---|
| Average Molecular Weight | 142.0697 |
|---|
| Monoisotopic Molecular Weight | 142.001456562 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrimidones. Pyrimidones are compounds that contain a pyrimidine ring, which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Pyrimidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidone
- Hydropyrimidine
- 2,5-dihydropyrimidine
- Ketone
- Carbonic acid derivative
- Cyclic ketone
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 1338AB |
|---|