| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:11 UTC |
|---|
| Update date | 2017-01-19 02:36:33 UTC |
|---|
| FoodComEx ID | PC000715 |
|---|
| FoodDB Record | FDB022978 |
|---|
| Chemical Information |
|---|
| Name | 2,4-Diaminobutyric acid |
|---|
| Description | 2,4-Diaminobutyric acid is a non-physiological, cationic amino acid analogue that is transported into cells by System A with potent antitumoral activity in vitro against human glioma cells, the result of the pronounced concentrated uptake of DAB in glioma cells to the extent that a cellular lysis could occur due to osmotic reasons. (PMID: 1561943) [HMDB] |
|---|
| CAS Number | 305-62-4 |
|---|
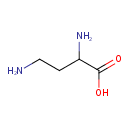
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (RS)-2,4-Diaminobutyric acid | hmdb | | 2,4-diamino-butanoate | hmdb | | 2,4-diamino-butanoic acid | hmdb | | 2,4-diamino-butyric acid | hmdb | | 2,4-Diamino-n-butyric acid | hmdb | | 2,4-Diaminobutanoate | hmdb | | 2,4-Diaminobutanoic acid | hmdb | | 2,4-Diaminobutyrate | hmdb | | a,g-Diaminobutanoate | Generator | | a,g-Diaminobutanoic acid | Generator | | alpha,gamma-Diaminobutanoate | Generator | | alpha,gamma-Diaminobutanoic acid | ChEBI | | alpha,gamma-Diaminobutyrate | hmdb | | alpha,gamma-Diaminobutyric acid | hmdb | | DL-2,4-Diamino-n-butyric acid | hmdb | | DL-2,4-Diaminobutanoate | hmdb | | DL-2,4-Diaminobutanoic acid | hmdb | | DL-2,4-Diaminobutyric acid | hmdb | | DL-alpha,gamma-Diaminobutyric acid | hmdb | | α,γ-diaminobutanoate | Generator | | α,γ-diaminobutanoic acid | Generator |
|
|---|
| Chemical Formula | C4H10N2O2 |
|---|
| IUPAC name | 2,4-diaminobutanoic acid |
|---|
| InChI Identifier | InChI=1S/C4H10N2O2/c5-2-1-3(6)4(7)8/h3H,1-2,5-6H2,(H,7,8) |
|---|
| InChI Key | OGNSCSPNOLGXSM-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NCCC(N)C(O)=O |
|---|
| Average Molecular Weight | 118.1344 |
|---|
| Monoisotopic Molecular Weight | 118.074227574 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Amino fatty acid
- Fatty acid
- Fatty acyl
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Primary aliphatic amine
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 600 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 3213AB |
|---|