| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:06 UTC |
|---|
| Update date | 2017-01-19 02:36:33 UTC |
|---|
| FoodComEx ID | PC000709 |
|---|
| FoodDB Record | FDB000939 |
|---|
| Chemical Information |
|---|
| Name | 1H-Indole-3-methanol |
|---|
| Description | Produced from glucosinolates in Brassica species on crushing or cooking. Potential nutriceutical
16082211).; Indole-3-carbinol is produced by members of the family Cruciferae and particularly members of the genus Brassica, for example, cabbage, radishes, cauliflower, broccoli, Brussels sprouts, and daikon). Indole-3-carbinol is metabolized to a number of products, including the dimeric 3,3'-diindolylmethane. Both 3,3'-diindolylmethane and Indole-3-carbinol are thought to have biological effects. Indole-3-carbinol is a natural chemopreventive compound. It has multiple anticarcinogenic and antitumorigenic properties by suppressing the proliferation of certain cancer cells, including breast cancer, prostate cancer, endometrial cancer, colon cancer, and leukemic cells (PMID: 16634522. 1H-Indole-3-methanol is found in many foods, some of which are broccoli, brassicas, cabbage, and brussel sprouts. |
|---|
| CAS Number | 700-06-1 |
|---|
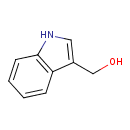
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1H-indol-3-ylmethanol | biospider | | 1H-Indole-3-methanol (9CI) | biospider | | 3-(Hydroxymethyl)indole | biospider | | 3-Hydroxymethylindole | db_source | | 3-Indolecarbinol | biospider | | 3-Indolemethanol | biospider | | 3-indolylcarbinol | biospider | | 3-Indolylmethanol | biospider | | C9H9NO | biospider | | I3C cpd | biospider | | Indinol | biospider | | Indole-3-carbinol | db_source | | indole-3-methanol | biospider | | PREVENTION 4 (INDOLE-3-CARBINOL) | biospider |
|
|---|
| Chemical Formula | C9H9NO |
|---|
| IUPAC name | (1H-indol-3-yl)methanol |
|---|
| InChI Identifier | InChI=1S/C9H9NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-5,10-11H,6H2 |
|---|
| InChI Key | IVYPNXXAYMYVSP-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OCC1=CNC2=CC=CC=C12 |
|---|
| Average Molecular Weight | 147.1739 |
|---|
| Monoisotopic Molecular Weight | 147.068413915 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 3-alkylindoles. 3-Alkylindoles are compounds containing an indole moiety that carries an alkyl chain at the 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indoles |
|---|
| Direct Parent | 3-alkylindoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-alkylindole
- Substituted pyrrole
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Azacycle
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Aromatic alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 158° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 50 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | A227 |
|---|
| Cayman Chemical | 11325 |
|---|
| Toronto Research Chemicals | I578000 |
|---|