| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:58 UTC |
|---|
| Update date | 2017-01-19 02:36:33 UTC |
|---|
| FoodComEx ID | PC000690 |
|---|
| FoodDB Record | FDB001493 |
|---|
| Chemical Information |
|---|
| Name | Cadaverine |
|---|
| Description | Cadaverine is a foul-smelling diamine formed by bacterial decarboxylation of lysine that occurs during protein hydrolysis during putrefaction of animal tissue. However, this diamine is not purely associated with putrefaction. It is also produced in small quantities by mammals. In particular, it is partially responsible for the distinctive smell of urine and semen. Elevated levels of cadaverine have been found in the urine of some patients with defects in lysine metabolism. Cadaverine is toxic in large doses. In rats it had a low acute oral toxicity of more than 2000 mg/kg body weight .; Cadaverine is a foul-smelling molecule produced by protein hydrolysis during putrefaction of animal tissue. Cadaverine is a toxic diamine with the formula NH2(CH2)5NH2, which is similar to putrescine. Cadaverine is also known by the names 1,5-pentanediamine and pentamethylenediamine. |
|---|
| CAS Number | 462-94-2 |
|---|
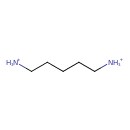
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1,5-Amylene diamine | biospider | | 1,5-Diamino-n-pentane | biospider | | 1,5-Diaminopentane | biospider | | 1,5-Diaminopentane dihydrochloride | biospider | | 1,5-Pentamethylenediamine | biospider | | 1,5-Pentanediamine | biospider | | Alpha,omega-pentanediamine | biospider | | Animal conIIne | biospider | | BioDex 1- | biospider | | Cadaverin | biospider | | Cadaverine dihydrochloride | biospider | | DAPE | ChEBI | | Diaminopentane | biospider | | H2N(CH2)5NH2 | biospider | | Pentamethylenediamine | biospider | | Pentamethylenediamine dihydrochloride | biospider | | Pentane-1,5-diamine | biospider |
|
|---|
| Chemical Formula | C5H16N2 |
|---|
| IUPAC name | pentane-1,5-bis(aminium) |
|---|
| InChI Identifier | InChI=1S/C5H14N2/c6-4-2-1-3-5-7/h1-7H2/p+2 |
|---|
| InChI Key | VHRGRCVQAFMJIZ-UHFFFAOYSA-P |
|---|
| Isomeric SMILES | [NH3+]CCCCC[NH3+] |
|---|
| Average Molecular Weight | 104.1939 |
|---|
| Monoisotopic Molecular Weight | 104.131348522 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as monoalkylamines. These are organic compounds containing an primary aliphatic amine group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Monoalkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organopnictogen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic cation
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | 9 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | C058000 |
|---|