| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:58 UTC |
|---|
| Update date | 2017-01-19 02:36:33 UTC |
|---|
| FoodComEx ID | PC000689 |
|---|
| FoodDB Record | FDB000371 |
|---|
| Chemical Information |
|---|
| Name | Erythritol |
|---|
| Description | Bulk sweetener with good taste props. Not metabolised, excreted unchanged in urine. Less sweet than sucrose. Use not yet permitted in most countries (1997). GRAS status for use as a sweetener, thickener, stabiliser, humectant, etc. in food
Erythritol ((2R,3S)-butane-1,2,3,4-tetraol) is a natural sugar alcohol (a type of sugar substitute) which has been approved for use in the United States and throughout much of the world. It occurs naturally in fruits and fermented foods. At industrial level, it is produced from glucose by fermentation with a yeast, Moniliella pollinis. It is 60?70% as sweet as table sugar yet it is almost non-caloric, does not affect blood sugar, does not cause tooth decay, and is absorbed by the body, therefore unlikely to cause gastric side effects unlike other sugar alcohols. Under U.S. Food and Drug Administration (FDA) labeling requirements, it has a caloric value of 0.2 calories per gram (95% less than sugar and other carbohydrates), though nutritional labelling varies from country to country?some countries like Japan label it as zero-calorie, while European Union regulations currently label it and all other sugar alcohols at 2.4 kcal/g.; Erythritol occurs widely in nature and has been found to occur naturally in several foods including wine, sake, beer, water melon, pear, grape and soy sauce. Evidence indicates that erythritol also exists endogenously in the tissues and body fluids of humans and animals. Erythritol is absorbed from the proximal intestine by passive diffusion in a manner similar to that of many low molecular weight organic molecules which do not have associated active transport systems, the rate of absorption being related to their molecular size; In the body, erythritol is absorbed into the bloodstream in the small intestine, and then for the most part excreted unchanged in the urine. Because erythritol is normally absorbed before it enters the large intestine, it does not normally cause laxative effects as are often experienced after over-consumption of other sugar alcohols (such as xylitol and maltitol) and most people will consume erythritol with no side effects. This is a unique characteristic, as other sugar alcohols are not absorbed directly by the body in this manner, and consequently are more prone to causing gastric distress.; erythritol, a 4-carbon molecule, passes through the intestinal membranes at a faster rate than larger molecules such as mannitol or glucose. In diabetics, erythritol also has been shown to be rapidly absorbed and excreted unchanged in the urine. Following absorption, ingested erythritol is rapidly distributed throughout the body and has been reported to occur in hepatocytes, pancreatic cells, and vascular smooth muscle cells. Erythritol also has been reported to cross the human placenta and to pass slowly from the plasma into the brain and cerebrospinal fluid. (PMID: 9862657, Food and Chemical Toxicology (1998), 36(12), 1139-1174.). Erythritol is found in caraway and garden tomato. |
|---|
| CAS Number | 149-32-6 |
|---|
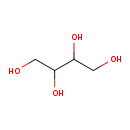
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2R,3S)-Butane-1,2,3,4-tetrol | ChEBI | | (2S,3S)-butane-1,2,3,4-tetrol | biospider | | (R*,S*)-1,2,3,4-Butanetetrol, 9CI, 8CI | db_source | | 1,2,3, 4-Tetrahydroxybutane | biospider | | 1,2,3,4-Butanetetrol | biospider | | 1,2,3,4-Butanetetrol, (2R,3S)-rel- | biospider | | 1,2,3,4-Butanetetrol, (R*,S*)- | biospider | | 1,2,3,4-Butanetetrol, (theta,S)- | biospider | | 1,2,3,4-Butanetetrol, [S-(R*,R*)]- | biospider | | 1,2,3,4-Tetrahydroxybutane | biospider | | 2(R),3(S)-1,2,3,4-Butanetetrol | biospider | | Antierythrite | biospider | | butane-1,2,3,4-tetrol | biospider | | Butanetetrol | biospider | | C*eridex | biospider | | D-erythritol | biospider | | D-treitol | biospider | | DL-1,2,3,4-Butanetetrol | biospider | | DL-threitol | biospider | | Erythrit | biospider | | Erythrite | biospider | | Erythritol, meso- | biospider | | erythro-Tetritol | ChEBI | | Erythroglucin | db_source | | Erythrol | biospider | | Erythrol (van) | biospider | | I-erythritol | biospider | | L-(-)-threitol | biospider | | L-erythritol | biospider | | L-threitol | biospider | | Lichen sugar | biospider | | Meso-erythritol | biospider | | Meso-eythritol | biospider | | Mesoerythritol | db_source | | Paycite | biospider | | Phycite | biospider | | Phycitol | db_source | | Tetrahydroxybutane | biospider |
|
|---|
| Chemical Formula | C4H10O4 |
|---|
| IUPAC name | butane-1,2,3,4-tetrol |
|---|
| InChI Identifier | InChI=1S/C4H10O4/c5-1-3(7)4(8)2-6/h3-8H,1-2H2 |
|---|
| InChI Key | UNXHWFMMPAWVPI-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OCC(O)C(O)CO |
|---|
| Average Molecular Weight | 122.1198 |
|---|
| Monoisotopic Molecular Weight | 122.057908808 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sugar alcohols. These are hydrogenated forms of carbohydrate in which the carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sugar alcohol
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -2.29 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 610 mg/mL at 22 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 121.5° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 20 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | W1905 |
|---|
| Glentham | GC1722 |
|---|
| MetaSci | HMDB0002994 |
|---|
| Tokyo Chemical Industry | HMDB0002994 |
|---|
| Toronto Research Chemicals | E650100 |
|---|