| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:56 UTC |
|---|
| Update date | 2017-01-19 02:36:32 UTC |
|---|
| FoodComEx ID | PC000684 |
|---|
| FoodDB Record | FDB022811 |
|---|
| Chemical Information |
|---|
| Name | Chlorate |
|---|
| Description | The chlorate anion has the formula ClO3−. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a roman numeral in parenthesis, e.g. chlorate(VII), refers to a particular oxyanion of chlorine.
As predicted by VSEPR, chlorate anions have trigonal pyrimidal structures.
Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Chlorates were once widely used in pyrotechnics, though their use has fallen due to their instability. Most pyrotechnic applications which used chlorates in the past now use perchlorates instead(From WIKI).
Chlorates are inorganic salts of chloric acid that contain the ClO3- ion.
Chlorate is a selective inhibitor of the synthesis of the high energy donor of sulfate 3'-phosphoadenosine 5'-phosphosulfate (PAPS). High endothelial venules (HEVs) are specialized post-capillary venules found in lymphoid tissues, that support high levels of lymphocyte extravasation from the blood; sulfation is key to the uniqueness of the HEV ligands and PAPS synthesis is required for sulfation. (PMID: 9498065)
Chlorate is a sulfate analogue that has been found to be a potent and nontoxic inhibitor of sulfation. Tyrosine sulfation is a widespread posttranslational modification that occurs in the trans Golgi in a reaction catalyzed by tyrosylprotein sulfotransferase. Tyrosine sulfation has been found to be irreversible, resulting in a life-long alteration in the phenotype of the secretory proteins. The intracellular transport kinetics of a secretory protein and the biological activity of certain neuropeptides have been found to be affected by this modification. (PMID: 3288098)
Na chlorate intoxication can occur mainly through poisoning by herbicides containing chlorate salts. (PMID: 10831921, 2239069) [HMDB] |
|---|
| CAS Number | 14866-68-3 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| [ClO3](-) | ChEBI | | Chlate | Generator | | CHLate ion | Generator | | Chlic acid | Generator | | CHLic acid ion | Generator | | Chlorate ion | hmdb | | Chloric acid | hmdb | | CHLORic acid ion | Generator | | Chlorine oxide | HMDB | | ClO3(-) | ChEBI |
|
|---|
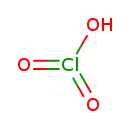
| Chemical Formula | ClHO3 |
|---|
| IUPAC name | chloric acid |
|---|
| InChI Identifier | InChI=1S/ClHO3/c2-1(3)4/h(H,2,3,4) |
|---|
| InChI Key | XTEGARKTQYYJKE-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | O[Cl](=O)=O |
|---|
| Average Molecular Weight | 84.459 |
|---|
| Monoisotopic Molecular Weight | 83.961421605 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of inorganic compounds known as non-metal chlorates. These are inorganic non-metallic compounds containing a chlorate as its largest oxoanion. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Non-metal oxoanionic compounds |
|---|
| Sub Class | Non-metal chlorates |
|---|
| Direct Parent | Non-metal chlorates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Non-metal chlorate
- Inorganic oxide
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 600 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |