| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:50 UTC |
|---|
| Update date | 2017-01-19 02:36:32 UTC |
|---|
| FoodComEx ID | PC000664 |
|---|
| FoodDB Record | FDB023231 |
|---|
| Chemical Information |
|---|
| Name | D-Pantethine |
|---|
| Description | Pantethine (Bis-pantethine) or "Co-enzyme pantethine" is a dimeric form of vitamin B5, composed of two molecules of pantothenic acid linked by cysteamine bridging groups. The monomer of this compound is known as pantetheine and is an intermediate in the production of Coenzyme A by the body. Pantethine is considered the more biologically active form of vitamin B5, but is less stable than pantothenic acid and tends to decompose over time if it is not kept refrigerated, so most vitamin B5 supplements are in the form of calcium pantothenate. [HMDB] |
|---|
| CAS Number | 16816-67-4 |
|---|
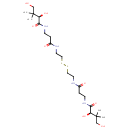
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (R-(R*,r*))-N,n'-(dithiobis(ethyleneimino(3-oxopropane-3,1-diyl)))bis(2,4-dihydroxy-3,3-dimethylbutyramide) | ChEBI | | Bis(pantothenamidoethyl) disulfide | ChEBI | | Bis(pantothenamidoethyl) disulphide | Generator | | D-Bis(N-pantothenyl-b-aminoethyl) disulfide | Generator | | D-Bis(N-pantothenyl-b-aminoethyl) disulphide | Generator | | D-Bis(N-pantothenyl-beta-aminoethyl) disulfide | ChEBI | | D-Bis(N-pantothenyl-beta-aminoethyl) disulphide | Generator | | D-Bis(N-pantothenyl-β-aminoethyl) disulfide | Generator | | D-Bis(N-pantothenyl-β-aminoethyl) disulphide | Generator | | N-[2-[2-[2-[3-[(2,4-Dihydroxy-3,3-dimethyl-butanoyl)amino]propanoylamino]ethyldisulfanyl]ethylcarbamoyl]ethyl]-2,4-dihydroxy-3,3-dimethyl-butanamide | ChEBI | | N-[2-[2-[2-[3-[(2,4-Dihydroxy-3,3-dimethyl-butanoyl)amino]propanoylamino]ethyldisulphanyl]ethylcarbamoyl]ethyl]-2,4-dihydroxy-3,3-dimethyl-butanamide | Generator | | Pantethine | hmdb | | Pantethine (JP15) | hmdb | | Pantetina | ChEBI | | Pantomin | ChEBI | | Pantosin | HMDB | | Pantosin (TN) | hmdb |
|
|---|
| Chemical Formula | C22H42N4O8S2 |
|---|
| IUPAC name | (2R)-N-[2-({2-[(2-{3-[(2R)-2,4-dihydroxy-3,3-dimethylbutanamido]propanamido}ethyl)disulfanyl]ethyl}carbamoyl)ethyl]-2,4-dihydroxy-3,3-dimethylbutanamide |
|---|
| InChI Identifier | InChI=1S/C22H42N4O8S2/c1-21(2,13-27)17(31)19(33)25-7-5-15(29)23-9-11-35-36-12-10-24-16(30)6-8-26-20(34)18(32)22(3,4)14-28/h17-18,27-28,31-32H,5-14H2,1-4H3,(H,23,29)(H,24,30)(H,25,33)(H,26,34)/t17-,18-/m0/s1 |
|---|
| InChI Key | DJWYOLJPSHDSAL-ROUUACIJSA-N |
|---|
| Isomeric SMILES | CC(C)(CO)[C@@H](O)C(=O)NCCC(=O)NCCSSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)CO |
|---|
| Average Molecular Weight | 554.721 |
|---|
| Monoisotopic Molecular Weight | 554.24440572 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Beta amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta amino acid or derivatives
- Fatty amide
- Monosaccharide
- N-acyl-amine
- Fatty acyl
- Carboxamide group
- Organic disulfide
- Secondary alcohol
- Secondary carboxylic acid amide
- Dialkyldisulfide
- Sulfenyl compound
- Primary alcohol
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Alcohol
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 10 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | Z3668 |
|---|
| Glentham | GP3689 |
|---|