| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:49 UTC |
|---|
| Update date | 2017-01-19 02:36:32 UTC |
|---|
| FoodComEx ID | PC000660 |
|---|
| FoodDB Record | FDB008310 |
|---|
| Chemical Information |
|---|
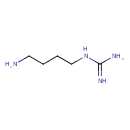
| Name | Agmatine |
|---|
| Description | Agmatine ((4-aminobutyl)guanidine, NH2-CH2-CH2-CH2-CH2-NH-C(-NH2)(=NH)) is the decarboxylation product of the amino acid arginine and is an intermediate in polyamine biosynthesis. It is a putative neurotransmitter. It is synthesized in the brain, stored in synaptic vesicles, accumulated by uptake, released by membrane depolarization, and inactivated by agmatinase. Agmatine binds to 2-adrenergic receptor and imidazoline binding sites, and blocks NMDA receptors and other cation ligand-gated channels. Agmatine inhibits nitric oxide synthase (NOS), and induces the release of some peptide hormones. Treatment with exogenous agmatine exerts neuroprotective effects in animal models of neurotrauma. -- Wikipedia; Agmatine ((4-aminobutyl)guanidine, NH2-CH2-CH2-CH2-CH2-NH-C(-NH2)(=NH)) is the decarboxylation product of the amino acid arginine and is an intermediate in polyamine biosynthesis. It is discussed as a putative neurotransmitter. It is synthesized in the brain, stored in synaptic vesicles, accumulated by uptake, released by membrane depolarization, and inactivated by agmatinase. Agmatine binds to ?2-adrenergic receptor and imidazoline binding sites, and blocks NMDA receptors and other cation ligand-gated channels. Agmatine inhibits nitric oxide synthase (NOS), and induces the release of some peptide hormones. Agmatine is found in many foods, some of which are fruits, kohlrabi, carob, and burdock. |
|---|
| CAS Number | 306-60-5 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (4-aminobutyl)-Guanidine | biospider | | (4-Aminobutyl)guanidine | ChEBI | | (4-Aminobutyl)guanidinium sulphate | biospider | | 1-(4-Aminobutyl)guanidine | biospider | | 1-Amino-4-guanidinobutane | db_source | | 1-Amino-4-guanidobutane | biospider | | 1,4-Butanediamine, N-(aminoiminomethyl)- | biospider | | 4-Guanidino-1-butanamine | biospider | | 4-Guanidinobutylamine | db_source | | Agmathine | biospider | | Agmatine | db_source | | Agmatine sulfate | biospider | | Agmatinium | HMDB | | Argmatine | biospider | | Guanidine, (4-aminobutyl)- | biospider | | Guanidine, (4-aminobutyl)- (8CI) | biospider | | Guanidine, (4-aminobutyl)- (8CI)(9CI) | biospider | | Guanidine, (4-aminobutyl)-, sulfate (1:1) | biospider | | N-(4-Amino-butyl)-guanidine | biospider | | N-(4-Aminobutyl)guanidine | biospider | | N-(aminoiminomethyl)-1,4-Butanediamine | biospider | | N-4-Aminobutylguanidine | biospider |
|
|---|
| Chemical Formula | C5H14N4 |
|---|
| IUPAC name | N-(4-aminobutyl)guanidine |
|---|
| InChI Identifier | InChI=1S/C5H14N4/c6-3-1-2-4-9-5(7)8/h1-4,6H2,(H4,7,8,9) |
|---|
| InChI Key | QYPPJABKJHAVHS-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NCCCCNC(N)=N |
|---|
| Average Molecular Weight | 130.1915 |
|---|
| Monoisotopic Molecular Weight | 130.121846468 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as guanidines. Guanidines are compounds containing a guanidine moiety, with the general structure (R1R2N)(R3R4N)C=N-R5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Guanidines |
|---|
| Direct Parent | Guanidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Guanidine
- Carboximidamide
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Primary aliphatic amine
- Imine
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 101.5-103° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |