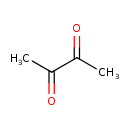
| Description | Diacetyl, also known as 2,3-butanedione or dimethylglyoxal, is a member of the class of compounds known as alpha-diketones. Alpha-diketones are organic compounds containing two ketone groups on two adjacent carbon atoms. Thus, diacetyl is considered to be an oxygenated hydrocarbon lipid molecule. Diacetyl is soluble (in water) and an extremely weak acidic compound (based on its pKa). Diacetyl is a strong, sweet, and butter tasting compound and can be found in a number of food items such as durian, italian sweet red pepper, giant butterbur, and millet, which makes diacetyl a potential biomarker for the consumption of these food products. Diacetyl can be found primarily in feces and saliva, as well as in human gonads, neuron and skeletal muscle tissues. Diacetyl exists in all living species, ranging from bacteria to humans. Diacetyl is a non-carcinogenic (not listed by IARC) potentially toxic compound. Diacetyl (IUPAC systematic name: butanedione or butane-2,3-dione) is an organic compound with the chemical formula (CH3CO)2. It is a yellow or green liquid with an intensely buttery flavor. It is a vicinal diketone (two C=O groups, side-by-side) with the molecular formula C4H6O2. Diacetyl occurs naturally in alcoholic beverages and is added to some foods to impart its buttery flavor . If the compound has been ingested, rapid gastric lavage should be performed using 5% sodium bicarbonate. For skin contact, the skin should be washed with soap and water. If the compound has entered the eyes, they should be washed with large quantities of isotonic saline or water. In serious cases, atropine and/or pralidoxime should be administered. Anti-cholinergic drugs work to counteract the effects of excess acetylcholine and reactivate AChE. Atropine can be used as an antidote in conjunction with pralidoxime or other pyridinium oximes (such as trimedoxime or obidoxime), though the use of '-oximes' has been found to be of no benefit, or possibly harmful, in at least two meta-analyses. Atropine is a muscarinic antagonist, and thus blocks the action of acetylcholine peripherally (T3DB). |
|---|