| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:47 UTC |
|---|
| Update date | 2017-01-19 02:36:31 UTC |
|---|
| FoodComEx ID | PC000653 |
|---|
| FoodDB Record | FDB022857 |
|---|
| Chemical Information |
|---|
| Name | 2,4-Diamino-6-hydroxypyrimidine |
|---|
| Description | 2,4-Diamino-6-hydroxypyrimidine (DAHP) is a selective inhibitor of GTP cyclohydrolase I (GTPCH) that restricts the de novo synthesis of tetrahydrobiopterin (BH4) or the BH4 precursor in vascular smooth muscle cells (VSMC). (PMID 12883322)
2,4-Diamino-6-hydroxypyrimidine also inhibits nitric oxide (NO) in both Interferon-gamma (IFN-gamma) and antigen (Ag)/IgE (Ag/IgE) systems, increasing Mast cells (MC) degranulation. (PMID 14514683)
Sepiapterin, a precursor to tetrahydrobiopterin in the salvage pathway, completely reverses the effect of 2,4-diamino-6-hydroxypyrimidine on neuronal NO-synthase (nNOS) ubiquitylation. (PMID 16216381) [HMDB] |
|---|
| CAS Number | 56-06-4 |
|---|
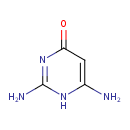
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2,4-Diamino-6-hydroxypyrimidine | hmdb | | 2,4-Diamino-6-pyrimidinone | hmdb | | 2,6-Diamino-4-hydroxypyrimidine | hmdb | | 2,6-Diamino-4-pyrimidinol | hmdb | | 2,6-Diamino-4(1H)-pyrimidinone | hmdb | | 2,6-Diamino-4(3H)-pyrimidinone | hmdb | | 2,6-Diaminopyrimidin-4-one | hmdb | | 6-Aminoisocytosine | hmdb | | 6-Hydroxy-2,4-pyrimidinediamine | hmdb |
|
|---|
| Chemical Formula | C4H6N4O |
|---|
| IUPAC name | 2,6-diamino-1,4-dihydropyrimidin-4-one |
|---|
| InChI Identifier | InChI=1S/C4H6N4O/c5-2-1-3(9)8-4(6)7-2/h1H,(H5,5,6,7,8,9) |
|---|
| InChI Key | SWELIMKTDYHAOY-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=CC(=O)N=C(N)N1 |
|---|
| Average Molecular Weight | 126.1166 |
|---|
| Monoisotopic Molecular Weight | 126.054160834 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrimidones. Pyrimidones are compounds that contain a pyrimidine ring, which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Pyrimidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminopyrimidine
- Pyrimidone
- Hydropyrimidine
- Vinylogous amide
- Heteroaromatic compound
- Azacycle
- Amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | D423 |
|---|
| AKSci | J92611 |
|---|
| Cayman Chemical | 81260 |
|---|
| Toronto Research Chemicals | D416353 |
|---|